[English] 日本語
 Yorodumi
Yorodumi- PDB-6dv6: Structure of the Salmonella SPI-1 type III secretion injectisome ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6dv6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
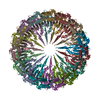
| Title | Structure of the Salmonella SPI-1 type III secretion injectisome secretin InvG (residues 176-end) in the open gate state | |||||||||
 Components Components | Protein InvG | |||||||||
 Keywords Keywords | MEMBRANE PROTEIN / Type III secretion system / secretin | |||||||||
| Function / homology |  Function and homology information Function and homology informationtype III protein secretion system complex / type II protein secretion system complex / protein secretion by the type III secretion system / protein secretion / cell outer membrane / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Hu, J. / Worrall, L.J. / Vuckovic, M. / Atkinson, C.E. / Strynadka, N.C.J. | |||||||||
| Funding support |  Canada, Canada,  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2018 Journal: Nat Commun / Year: 2018Title: Cryo-EM analysis of the T3S injectisome reveals the structure of the needle and open secretin. Authors: J Hu / L J Worrall / C Hong / M Vuckovic / C E Atkinson / N Caveney / Z Yu / N C J Strynadka /   Abstract: The bacterial type III secretion system, or injectisome, is a syringe shaped nanomachine essential for the virulence of many disease causing Gram-negative bacteria. At the core of the injectisome ...The bacterial type III secretion system, or injectisome, is a syringe shaped nanomachine essential for the virulence of many disease causing Gram-negative bacteria. At the core of the injectisome structure is the needle complex, a continuous channel formed by the highly oligomerized inner and outer membrane hollow rings and a polymerized helical needle filament which spans through and projects into the infected host cell. Here we present the near-atomic resolution structure of a needle complex from the prototypical Salmonella Typhimurium SPI-1 type III secretion system, with local masking protocols allowing for model building and refinement of the major membrane spanning components of the needle complex base in addition to an isolated needle filament. This work provides significant insight into injectisome structure and assembly and importantly captures the molecular basis for substrate induced gating in the giant outer membrane secretin portal family. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6dv6.cif.gz 6dv6.cif.gz | 895.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6dv6.ent.gz pdb6dv6.ent.gz | 714.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6dv6.json.gz 6dv6.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dv/6dv6 https://data.pdbj.org/pub/pdb/validation_reports/dv/6dv6 ftp://data.pdbj.org/pub/pdb/validation_reports/dv/6dv6 ftp://data.pdbj.org/pub/pdb/validation_reports/dv/6dv6 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8915MC  8913C  8914C  8924C  6duzC  6dv3C  6dwbC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 61835.559 Da / Num. of mol.: 15 / Source method: isolated from a natural source Source: (natural)  Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium (bacteria)References: UniProt: P35672 |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Salmonella SPI-1 type III secretion injectisome secretin oligomer residues 176-end Type: COMPLEX / Entity ID: all / Source: NATURAL |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:  Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 40 e/Å2 / Detector mode: COUNTING / Film or detector model: FEI FALCON III (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.12_2829: / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 80000 | ||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C15 (15 fold cyclic) | ||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.9 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 34000 / Symmetry type: POINT |
 Movie
Movie Controller
Controller






 PDBj
PDBj
