[English] 日本語
 Yorodumi
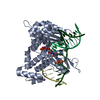
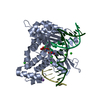
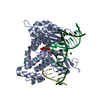
Yorodumi- PDB-5j2a: Ternary complex crystal structure of DNA polymerase Beta with A:C... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5j2a | ||||||
|---|---|---|---|---|---|---|---|
| Title | Ternary complex crystal structure of DNA polymerase Beta with A:C mismatch at the primer terminus | ||||||
 Components Components |
| ||||||
 Keywords Keywords | Transferase/DNA / DNA Polymerase Beta / mismatch extension / Ternary complex / Transferase-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology informationResolution of AP sites via the single-nucleotide replacement pathway / immunoglobulin heavy chain V-D-J recombination / Resolution of AP sites via the multiple-nucleotide patch replacement pathway / Abasic sugar-phosphate removal via the single-nucleotide replacement pathway / APEX1-Independent Resolution of AP Sites via the Single Nucleotide Replacement Pathway / Lyases; Carbon-oxygen lyases; Other carbon-oxygen lyases / pyrimidine dimer repair / POLB-Dependent Long Patch Base Excision Repair / homeostasis of number of cells / PCNA-Dependent Long Patch Base Excision Repair ...Resolution of AP sites via the single-nucleotide replacement pathway / immunoglobulin heavy chain V-D-J recombination / Resolution of AP sites via the multiple-nucleotide patch replacement pathway / Abasic sugar-phosphate removal via the single-nucleotide replacement pathway / APEX1-Independent Resolution of AP Sites via the Single Nucleotide Replacement Pathway / Lyases; Carbon-oxygen lyases; Other carbon-oxygen lyases / pyrimidine dimer repair / POLB-Dependent Long Patch Base Excision Repair / homeostasis of number of cells / PCNA-Dependent Long Patch Base Excision Repair / response to hyperoxia / 5'-deoxyribose-5-phosphate lyase activity / lymph node development / salivary gland morphogenesis / somatic hypermutation of immunoglobulin genes / spleen development / base-excision repair, gap-filling / DNA-(apurinic or apyrimidinic site) endonuclease activity / class I DNA-(apurinic or apyrimidinic site) endonuclease activity / DNA-(apurinic or apyrimidinic site) lyase / response to gamma radiation / spindle microtubule / base-excision repair / double-strand break repair via nonhomologous end joining / DNA-templated DNA replication / intrinsic apoptotic signaling pathway in response to DNA damage / neuron apoptotic process / microtubule binding / DNA-directed DNA polymerase / response to ethanol / in utero embryonic development / microtubule / damaged DNA binding / DNA-directed DNA polymerase activity / Ub-specific processing proteases / lyase activity / inflammatory response / DNA repair / DNA damage response / enzyme binding / protein-containing complex / nucleoplasm / metal ion binding / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)synthetic construct (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.5 Å MOLECULAR REPLACEMENT / Resolution: 2.5 Å | ||||||
 Authors Authors | Batra, V.K. / Wilson, S.H. | ||||||
 Citation Citation |  Journal: Structure / Year: 2016 Journal: Structure / Year: 2016Title: Structures of DNA Polymerase Mispaired DNA Termini Transitioning to Pre-catalytic Complexes Support an Induced-Fit Fidelity Mechanism. Authors: Batra, V.K. / Beard, W.A. / Pedersen, L.C. / Wilson, S.H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5j2a.cif.gz 5j2a.cif.gz | 108.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5j2a.ent.gz pdb5j2a.ent.gz | 76.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5j2a.json.gz 5j2a.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/j2/5j2a https://data.pdbj.org/pub/pdb/validation_reports/j2/5j2a ftp://data.pdbj.org/pub/pdb/validation_reports/j2/5j2a ftp://data.pdbj.org/pub/pdb/validation_reports/j2/5j2a | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5j0oC  5j0pC  5j0qC  5j0rC  5j0sC  5j0tC  5j0uC  5j0wC  5j0xC  5j0yC  5j29C  5j2bC  5j2cC  5j2dC  5j2eC  5j2fC  5j2gC  5j2hC  5j2iC  5j2jC  5j2kC  5tzvC  2fmsS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 38241.672 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: POLB / Plasmid: pWL-11 / Production host: Homo sapiens (human) / Gene: POLB / Plasmid: pWL-11 / Production host:  References: UniProt: P06746, DNA-directed DNA polymerase, Lyases; Carbon-oxygen lyases; Other carbon-oxygen lyases |
|---|
-DNA chain , 3 types, 3 molecules TPD
| #2: DNA chain | Mass: 4837.159 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
|---|---|
| #3: DNA chain | Mass: 3061.004 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
| #4: DNA chain | Mass: 1536.035 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
-Non-polymers , 5 types, 264 molecules 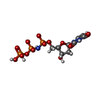








| #5: Chemical | ChemComp-DUP / | ||||||
|---|---|---|---|---|---|---|---|
| #6: Chemical | | #7: Chemical | #8: Chemical | #9: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.27 Å3/Da / Density % sol: 45.83 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop Details: 16-18% PEG 3350, 50 mM Imidazole, 350 mM Sodium Acetate PH range: 7.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU MICROMAX-007 HF / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU MICROMAX-007 HF / Wavelength: 1.5418 Å |
| Detector | Type: RIGAKU SATURN 92 / Detector: CCD / Date: Mar 8, 2006 / Details: Viramax |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.5→50 Å / Num. obs: 14492 / % possible obs: 99.7 % / Redundancy: 3.2 % / Rmerge(I) obs: 0.114 / Net I/σ(I): 12.8 |
| Reflection shell | Resolution: 2.5→2.59 Å / Redundancy: 3.1 % / Rmerge(I) obs: 0.564 / Mean I/σ(I) obs: 2 / % possible all: 99.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2FMS Resolution: 2.5→50 Å / Cross valid method: THROUGHOUT / σ(F): 0
| ||||||||||||||||||||||||
| Solvent computation | Bsol: 33.8768 Å2 | ||||||||||||||||||||||||
| Displacement parameters | Biso max: 67.06 Å2 / Biso mean: 27.1632 Å2 / Biso min: 1 Å2
| ||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.5→50 Å
| ||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.5→2.66 Å
| ||||||||||||||||||||||||
| Xplor file | Serial no: 1 / Param file: ACU.param |
 Movie
Movie Controller
Controller



















 PDBj
PDBj












































