[English] 日本語
 Yorodumi
Yorodumi- PDB-5g6g: Structure of Bacillus subtilis Nitric Oxide Synthase in complex w... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5g6g | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Bacillus subtilis Nitric Oxide Synthase in complex with 7-((2-((Methylamino)methyl)phenoxy)methyl)quinolin-2-amine | |||||||||
 Components Components | NITRIC OXIDE SYNTHASE OXYGENASE | |||||||||
 Keywords Keywords | OXIDOREDUCTASE / NITRIC OXIDE SYNTHASE / INHIBITOR | |||||||||
| Function / homology |  Function and homology information Function and homology informationnitric-oxide synthase (flavodoxin) / nitric-oxide synthase activity / nitric oxide biosynthetic process / heme binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.981 Å MOLECULAR REPLACEMENT / Resolution: 1.981 Å | |||||||||
 Authors Authors | Holden, J.K. / Poulos, T.L. | |||||||||
 Citation Citation |  Journal: Biochemistry / Year: 2016 Journal: Biochemistry / Year: 2016Title: Targeting Bacterial Nitric Oxide Synthase with Aminoquinoline-Based Inhibitors. Authors: Holden, J.K. / Lewis, M.C. / Cinelli, M.A. / Abdullatif, Z. / Pensa, A.V. / Silverman, R.B. / Poulos, T.L. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5g6g.cif.gz 5g6g.cif.gz | 175 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5g6g.ent.gz pdb5g6g.ent.gz | 138.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5g6g.json.gz 5g6g.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/g6/5g6g https://data.pdbj.org/pub/pdb/validation_reports/g6/5g6g ftp://data.pdbj.org/pub/pdb/validation_reports/g6/5g6g ftp://data.pdbj.org/pub/pdb/validation_reports/g6/5g6g | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5g65C  5g66C  5g67C  5g68C  5g69C  5g6aC  5g6bC  5g6cC  5g6dC  5g6eC  5g6fC  5g6hC  5g6iC  5g6jC  5g6kC  5g6lC  5g6mC  5g6nC  5g6oC  5g6pC  5g6qC  4lwaS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 41787.082 Da / Num. of mol.: 1 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Non-polymers , 5 types, 228 molecules 
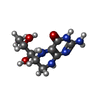

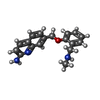





| #2: Chemical | ChemComp-HEM / |
|---|---|
| #3: Chemical | ChemComp-H4B / |
| #4: Chemical | ChemComp-CL / |
| #5: Chemical | ChemComp-H8B / |
| #6: Water | ChemComp-HOH / |
-Details
| Sequence details | SURFACE ENTROPY MUTATIONS E25A, E26A AND E316A |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.83 Å3/Da / Density % sol: 56.59 % Description: CC ONE HALF FOR HIGH RESOLUTION DATA SHELL AT 0.889. DIFFRACTION HAD STRONG ANISOTROPY AND RAW DATA WAS FURTHER SCALED USING THE DIFFRACTION ANISOTROPY SERVER. |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7.6 Details: 60 MM BIS-TRIS METHANE, 40 MM CITRIC ACID, 20% PEG3350, 1.9% 1-PROPANOL, PH 7.6, VAPOR DIFFUSION, HANGING DROP, TEMPERATURE 298K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 8.2.2 / Wavelength: 1 / Beamline: 8.2.2 / Wavelength: 1 |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jun 25, 2015 / Details: MIRRORS |
| Radiation | Monochromator: SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.98→37.59 Å / Num. obs: 33826 / % possible obs: 99.9 % / Observed criterion σ(I): -3 / Redundancy: 5.5 % / Biso Wilson estimate: 24.48 Å2 / Rmerge(I) obs: 0.15 / Net I/σ(I): 8.1 |
| Reflection shell | Resolution: 1.98→2.03 Å / Redundancy: 3.9 % / Rmerge(I) obs: 1.27 / Mean I/σ(I) obs: 0.9 / % possible all: 99.2 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4LWA Resolution: 1.981→37.592 Å / SU ML: 0.2 / σ(F): 1.35 / Phase error: 23.67 / Stereochemistry target values: ML Details: RAW DATA HAD STRONG ANISOTROPY AND DATA WAS FURTHER SCALED USING THE DIFFRACTION ANISOTROPY SERVER.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.981→37.592 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 5.5184 Å / Origin y: 19.7258 Å / Origin z: 22.3401 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: ALL |
 Movie
Movie Controller
Controller






















 PDBj
PDBj





