[English] 日本語
 Yorodumi
Yorodumi- PDB-5enc: Crystal structure of the second bromodomain of Pleckstrin homolog... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5enc | ||||||
|---|---|---|---|---|---|---|---|
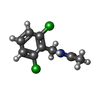
| Title | Crystal structure of the second bromodomain of Pleckstrin homology domain interacting protein (PHIP) in complex with N-(2,6-Dichlorobenzyl)acetamide (SGC - Diamond I04-1 fragment screening) | ||||||
 Components Components | PH-interacting protein | ||||||
 Keywords Keywords | SIGNALING PROTEIN / bromodomain / PHIP / crystallographic fragment screen / Structural Genomics / Structural Genomics Consortium / SGC / transcription | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of cell morphogenesis / positive regulation of insulin-like growth factor receptor signaling pathway / regulation of protein phosphorylation / histone reader activity / RHOBTB2 GTPase cycle / cytoskeleton organization / positive regulation of mitotic nuclear division / negative regulation of extrinsic apoptotic signaling pathway / insulin receptor binding / insulin receptor signaling pathway ...regulation of cell morphogenesis / positive regulation of insulin-like growth factor receptor signaling pathway / regulation of protein phosphorylation / histone reader activity / RHOBTB2 GTPase cycle / cytoskeleton organization / positive regulation of mitotic nuclear division / negative regulation of extrinsic apoptotic signaling pathway / insulin receptor binding / insulin receptor signaling pathway / regulation of cell shape / positive regulation of cell population proliferation / regulation of transcription by RNA polymerase II / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / positive regulation of transcription by RNA polymerase II / nucleus Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.59 Å molecular replacement / Resolution: 1.59 Å | ||||||
 Authors Authors | Krojer, T. / Talon, R. / Collins, P. / Bradley, A. / Cox, O. / Szykowska, A. / Burgess-Brown, N. / Brennan, P. / Bountra, C. / Arrowsmith, C.H. ...Krojer, T. / Talon, R. / Collins, P. / Bradley, A. / Cox, O. / Szykowska, A. / Burgess-Brown, N. / Brennan, P. / Bountra, C. / Arrowsmith, C.H. / Edwards, A. / von Delft, F. / Structural Genomics Consortium (SGC) | ||||||
 Citation Citation |  Journal: Chem Sci / Year: 2016 Journal: Chem Sci / Year: 2016Title: A poised fragment library enables rapid synthetic expansion yielding the first reported inhibitors of PHIP(2), an atypical bromodomain. Authors: Cox, O.B. / Krojer, T. / Collins, P. / Monteiro, O. / Talon, R. / Bradley, A. / Fedorov, O. / Amin, J. / Marsden, B.D. / Spencer, J. / von Delft, F. / Brennan, P.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5enc.cif.gz 5enc.cif.gz | 71.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5enc.ent.gz pdb5enc.ent.gz | 51.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5enc.json.gz 5enc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/en/5enc https://data.pdbj.org/pub/pdb/validation_reports/en/5enc ftp://data.pdbj.org/pub/pdb/validation_reports/en/5enc ftp://data.pdbj.org/pub/pdb/validation_reports/en/5enc | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5enbC  5eneC  5enfC  5enhC  5eniC  5enjC  3mb3S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 15594.661 Da / Num. of mol.: 1 / Fragment: Bromodomain, UNP residues 1315-1440 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PHIP, WDR11 / Plasmid: pNIC28-Bsa4 / Production host: Homo sapiens (human) / Gene: PHIP, WDR11 / Plasmid: pNIC28-Bsa4 / Production host:  | ||||
|---|---|---|---|---|---|
| #2: Chemical | | #3: Chemical | ChemComp-5QD / ~{ | #4: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.14 Å3/Da / Density % sol: 42.56 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 7.5 Details: 0.1M HEPES pH 7.5 , 0.15M magnesium chloride , 32% PEG3350 |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04-1 / Wavelength: 0.92001 Å / Beamline: I04-1 / Wavelength: 0.92001 Å | |||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 2M / Detector: PIXEL / Date: Oct 17, 2014 | |||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.92001 Å / Relative weight: 1 | |||||||||||||||||||||||||||
| Reflection | Resolution: 1.59→28.8 Å / Num. obs: 18820 / % possible obs: 99.8 % / Redundancy: 6.4 % / CC1/2: 0.999 / Rmerge(I) obs: 0.041 / Rpim(I) all: 0.018 / Net I/σ(I): 21.8 / Num. measured all: 120066 | |||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: _
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3MB3 Resolution: 1.59→28.8 Å / Cor.coef. Fo:Fc: 0.961 / Cor.coef. Fo:Fc free: 0.94 / SU B: 4.491 / SU ML: 0.081 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.101 / ESU R Free: 0.105 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : WITH TLS ADDED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 65.92 Å2 / Biso mean: 31.014 Å2 / Biso min: 19.06 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.59→28.8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.589→1.631 Å / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj