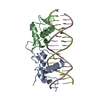
Entry Database : PDB / ID : 5.0E+69 Title Glucocorticoid receptor DNA binding domain - IL8 NF-kB response element complex DNA (5'-D(*AP*TP*CP*GP*TP*GP*GP*AP*AP*TP*TP*TP*CP*CP*TP*C)-3')DNA (5'-D(*GP*AP*GP*GP*AP*AP*AP*TP*TP*CP*CP*AP*CP*GP*AP*T)-3')Glucocorticoid receptor Keywords / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 1.85 Å Authors Hudson, W.H. / Rye, E.A. / Herbst, A.G. / Ortlund, E.A. Journal : Nat Commun / Year : 2018Title : Cryptic glucocorticoid receptor-binding sites pervade genomic NF-kappa B response elements.Authors : Hudson, W.H. / Vera, I.M.S. / Nwachukwu, J.C. / Weikum, E.R. / Herbst, A.G. / Yang, Q. / Bain, D.L. / Nettles, K.W. / Kojetin, D.J. / Ortlund, E.A. History Deposition Oct 9, 2015 Deposition site / Processing site Revision 1.0 Feb 8, 2017 Provider / Type Revision 1.1 Oct 24, 2018 Group / Database references / Category / citation_authorItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year Revision 1.2 Mar 6, 2024 Group / Database references / Derived calculationsCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / struct_conn Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.85 Å
MOLECULAR REPLACEMENT / Resolution: 1.85 Å  Authors
Authors Citation
Citation Journal: Nat Commun / Year: 2018
Journal: Nat Commun / Year: 2018 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5e69.cif.gz
5e69.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5e69.ent.gz
pdb5e69.ent.gz PDB format
PDB format 5e69.json.gz
5e69.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/e6/5e69
https://data.pdbj.org/pub/pdb/validation_reports/e6/5e69 ftp://data.pdbj.org/pub/pdb/validation_reports/e6/5e69
ftp://data.pdbj.org/pub/pdb/validation_reports/e6/5e69 Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: NR3C1, GRL / Production host:
Homo sapiens (human) / Gene: NR3C1, GRL / Production host: 
 Homo sapiens (human)
Homo sapiens (human) Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION
X-RAY DIFFRACTION Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 22-ID / Wavelength: 1 Å
/ Beamline: 22-ID / Wavelength: 1 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 1.85→34.267 Å / SU ML: 0.23 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 28.87 / Stereochemistry target values: ML
MOLECULAR REPLACEMENT / Resolution: 1.85→34.267 Å / SU ML: 0.23 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 28.87 / Stereochemistry target values: ML Movie
Movie Controller
Controller















 PDBj
PDBj
















































