[English] 日本語
 Yorodumi
Yorodumi- PDB-5dis: Crystal structure of a CRM1-RanGTP-SPN1 export complex bound to a... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5dis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of a CRM1-RanGTP-SPN1 export complex bound to a 113 amino acid FG-repeat containing fragment of Nup214 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | TRANSPORT PROTEIN / FG-repeats / Nucleoporin / Nup214 / Exportin | |||||||||
| Function / homology |  Function and homology information Function and homology informationcytoplasmic side of nuclear pore / cellular response to triglyceride / RNA import into nucleus / cellular response to salt / HuR (ELAVL1) binds and stabilizes mRNA / annulate lamellae / regulation of nucleocytoplasmic transport / regulation of proteasomal ubiquitin-dependent protein catabolic process / RNA cap binding / pre-miRNA export from nucleus ...cytoplasmic side of nuclear pore / cellular response to triglyceride / RNA import into nucleus / cellular response to salt / HuR (ELAVL1) binds and stabilizes mRNA / annulate lamellae / regulation of nucleocytoplasmic transport / regulation of proteasomal ubiquitin-dependent protein catabolic process / RNA cap binding / pre-miRNA export from nucleus / RNA nuclear export complex / snRNA import into nucleus / manchette / nuclear export signal receptor activity / regulation of centrosome duplication / cellular response to mineralocorticoid stimulus / Nuclear Pore Complex (NPC) Disassembly / Regulation of Glucokinase by Glucokinase Regulatory Protein / Defective TPR may confer susceptibility towards thyroid papillary carcinoma (TPC) / Transport of Ribonucleoproteins into the Host Nucleus / Regulation of cholesterol biosynthesis by SREBP (SREBF) / Transport of the SLBP independent Mature mRNA / Transport of the SLBP Dependant Mature mRNA / importin-alpha family protein binding / NS1 Mediated Effects on Host Pathways / SUMOylation of SUMOylation proteins / NLS-dependent protein nuclear import complex / structural constituent of nuclear pore / regulation of protein export from nucleus / Transport of Mature mRNA Derived from an Intronless Transcript / Rev-mediated nuclear export of HIV RNA / SUMOylation of RNA binding proteins / Nuclear import of Rev protein / protein localization to nucleolus / NEP/NS2 Interacts with the Cellular Export Machinery / Transport of Mature mRNA derived from an Intron-Containing Transcript / RNA export from nucleus / tRNA processing in the nucleus / Postmitotic nuclear pore complex (NPC) reformation / GTP metabolic process / nuclear import signal receptor activity / protein-containing complex localization / nucleocytoplasmic transport / nuclear localization sequence binding / MicroRNA (miRNA) biogenesis / Viral Messenger RNA Synthesis / DNA metabolic process / SUMOylation of ubiquitinylation proteins / Vpr-mediated nuclear import of PICs / detection of maltose stimulus / maltose transport complex / dynein intermediate chain binding / carbohydrate transport / Maturation of hRSV A proteins / protein complex oligomerization / mitotic sister chromatid segregation / SUMOylation of DNA replication proteins / viral process / carbohydrate transmembrane transporter activity / ribosomal large subunit export from nucleus / maltose binding / spermatid development / Regulation of HSF1-mediated heat shock response / Estrogen-dependent nuclear events downstream of ESR-membrane signaling / maltose transport / maltodextrin transmembrane transport / protein localization to nucleus / nuclear pore / sperm flagellum / mRNA export from nucleus / Cajal body / SUMOylation of DNA damage response and repair proteins / ribosomal subunit export from nucleus / Cyclin A/B1/B2 associated events during G2/M transition / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / NPAS4 regulates expression of target genes / ribosomal small subunit export from nucleus / Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal / cytoskeleton organization / Transcriptional and post-translational regulation of MITF-M expression and activity / centriole / Mitotic Prometaphase / ATP-binding cassette (ABC) transporter complex / EML4 and NUDC in mitotic spindle formation / protein export from nucleus / Resolution of Sister Chromatid Cohesion / SUMOylation of chromatin organization proteins / Downregulation of TGF-beta receptor signaling / mitotic spindle organization / HCMV Late Events / cell chemotaxis / male germ cell nucleus / hippocampus development / protein tetramerization / Deactivation of the beta-catenin transactivating complex / Heme signaling / Transcriptional regulation by small RNAs / RHO GTPases Activate Formins / recycling endosome / MAPK6/MAPK4 signaling Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.85 Å MOLECULAR REPLACEMENT / Resolution: 2.85 Å | |||||||||
 Authors Authors | Monecke, T. / Port, S.A. / Dickmanns, A. / Kehlenbach, R.H. / Ficner, R. | |||||||||
| Funding support |  Germany, 1items Germany, 1items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2015 Journal: Cell Rep / Year: 2015Title: Structural and Functional Characterization of CRM1-Nup214 Interactions Reveals Multiple FG-Binding Sites Involved in Nuclear Export. Authors: Port, S.A. / Monecke, T. / Dickmanns, A. / Spillner, C. / Hofele, R. / Urlaub, H. / Ficner, R. / Kehlenbach, R.H. #1:  Journal: To Be Published Journal: To Be PublishedTitle: Combining dehydration, construct optimization and improved data collection to solve the crystal structure of a CRM1-RanGTP-SPN1-Nup214 quaternary export complex Authors: Monecke, T. / Dickmanns, A. / Weiss, M.S. / Port, S.A. / Kehlenbach, R.H. / Ficner, R. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5dis.cif.gz 5dis.cif.gz | 788.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5dis.ent.gz pdb5dis.ent.gz | 642.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5dis.json.gz 5dis.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/di/5dis https://data.pdbj.org/pub/pdb/validation_reports/di/5dis ftp://data.pdbj.org/pub/pdb/validation_reports/di/5dis ftp://data.pdbj.org/pub/pdb/validation_reports/di/5dis | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 4 types, 4 molecules ABCD
| #1: Protein | Mass: 120395.742 Da / Num. of mol.: 1 / Fragment: UNP residues 5-1058 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: XPO1, CRM1 / Production host: Homo sapiens (human) / Gene: XPO1, CRM1 / Production host:  |
|---|---|
| #2: Protein | Mass: 19812.088 Da / Num. of mol.: 1 / Fragment: UNP residues 8-179 / Mutation: Q69L Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: RAN, ARA24, OK/SW-cl.81 / Production host: Homo sapiens (human) / Gene: RAN, ARA24, OK/SW-cl.81 / Production host:  |
| #3: Protein | Mass: 33276.527 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SNUPN, RNUT1, SPN1 / Production host: Homo sapiens (human) / Gene: SNUPN, RNUT1, SPN1 / Production host:  |
| #4: Protein | Mass: 50577.461 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) Homo sapiens (human)Gene: malE, b4034, JW3994, NUP214, CAIN, CAN, KIAA0023 / Production host:  |
-Sugars , 1 types, 1 molecules
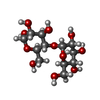
| #5: Polysaccharide | alpha-D-glucopyranose-(1-4)-alpha-D-glucopyranose / alpha-maltose |
|---|
-Non-polymers , 4 types, 16 molecules 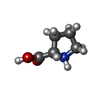






| #6: Chemical | | #7: Chemical | ChemComp-GTP / | #8: Chemical | ChemComp-MG / | #9: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.29 Å3/Da / Density % sol: 62.56 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 7.5 Details: 5% PEG 8000, 0.2M L-proline, 0.1M Tris pH 7.5, 4 mM D-maltose, 180 mM LiCl |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  BESSY BESSY  / Beamline: 14.1 / Wavelength: 0.9184 Å / Beamline: 14.1 / Wavelength: 0.9184 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Nov 28, 2014 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: Si111-DCM with sagital bender / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.9184 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.85→48.49 Å / Num. obs: 67922 / % possible obs: 98.2 % / Observed criterion σ(I): -3 / Redundancy: 4.6 % / Biso Wilson estimate: 80.01 Å2 / Rmerge F obs: 0.999 / Rmerge(I) obs: 0.055 / Rrim(I) all: 0.062 / Χ2: 1.037 / Net I/σ(I): 15.52 / Num. measured all: 313050 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: _
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3GJX, 1ANF Resolution: 2.85→48.485 Å / SU ML: 0.42 / Cross valid method: THROUGHOUT / σ(F): 1.35 / Phase error: 32.17 / Stereochemistry target values: ML Details: Overall, the electron density map for MBP and especially its N-terminal lobe is of poor quality. This suggests that significant movement of MBP in the crystal lattice is possible, which is ...Details: Overall, the electron density map for MBP and especially its N-terminal lobe is of poor quality. This suggests that significant movement of MBP in the crystal lattice is possible, which is consistent with the overall elevated B-factors of the MBP residues. Hence, several parts of MBP are not defined in the electron density map. Since dissolved crystals analysed by SDS-PAGE clearly showed the presence of full-length MBP in order to retain structural integrity of the crystal lattice and the MBP, the residues were not omitted.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 1 Å / VDW probe radii: 1.2 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.85→48.485 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj