[English] 日本語
 Yorodumi
Yorodumi- PDB-5cu2: Crystal structure of CK2alpha with 2-hydroxy-5-methylbenzoic acid... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5cu2 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of CK2alpha with 2-hydroxy-5-methylbenzoic acid and (methyl 4-((3-(3-chloro-4-(phenyl)benzylamino)propyl)amino)-4-oxobutanoat bound | ||||||
 Components Components | Casein kinase II subunit alpha | ||||||
 Keywords Keywords | TRANSFERASE / CK2alpha / CK2a / fragment based drug discovery / high concentration screening / selective ATP competitive inhibitors / surface entrophy reduction | ||||||
| Function / homology |  Function and homology information Function and homology informationPhosphorylation and nuclear translocation of the CRY:PER:kinase complex / Phosphorylation and nuclear translocation of BMAL1 (ARNTL) and CLOCK / Regulation of CDH1 posttranslational processing and trafficking to plasma membrane / positive regulation of aggrephagy / regulation of chromosome separation / WNT mediated activation of DVL / Condensation of Prometaphase Chromosomes / protein kinase CK2 complex / symbiont-mediated disruption of host cell PML body / Receptor Mediated Mitophagy ...Phosphorylation and nuclear translocation of the CRY:PER:kinase complex / Phosphorylation and nuclear translocation of BMAL1 (ARNTL) and CLOCK / Regulation of CDH1 posttranslational processing and trafficking to plasma membrane / positive regulation of aggrephagy / regulation of chromosome separation / WNT mediated activation of DVL / Condensation of Prometaphase Chromosomes / protein kinase CK2 complex / symbiont-mediated disruption of host cell PML body / Receptor Mediated Mitophagy / Sin3-type complex / Synthesis of PC / negative regulation of signal transduction by p53 class mediator / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / Maturation of hRSV A proteins / negative regulation of apoptotic signaling pathway / negative regulation of double-strand break repair via homologous recombination / positive regulation of Wnt signaling pathway / negative regulation of proteasomal ubiquitin-dependent protein catabolic process / Signal transduction by L1 / Hsp90 protein binding / PML body / Wnt signaling pathway / Regulation of PTEN stability and activity / kinase activity / positive regulation of protein catabolic process / KEAP1-NFE2L2 pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / rhythmic process / double-strand break repair / protein folding / positive regulation of cell growth / Regulation of TP53 Activity through Phosphorylation / non-specific serine/threonine protein kinase / regulation of cell cycle / negative regulation of translation / protein stabilization / protein serine kinase activity / protein serine/threonine kinase activity / apoptotic process / positive regulation of cell population proliferation / DNA damage response / signal transduction / nucleoplasm / ATP binding / identical protein binding / nucleus / plasma membrane / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.705 Å molecular replacement / Resolution: 1.705 Å | ||||||
 Authors Authors | Brear, P. / De Fusco, C. / Georgiou, K.H. / Spring, D. / Hyvonen, M. | ||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| ||||||
 Citation Citation |  Journal: Bioorg. Med. Chem. / Year: 2017 Journal: Bioorg. Med. Chem. / Year: 2017Title: A fragment-based approach leading to the discovery of a novel binding site and the selective CK2 inhibitor CAM4066. Authors: De Fusco, C. / Brear, P. / Iegre, J. / Georgiou, K.H. / Sore, H.F. / Hyvonen, M. / Spring, D.R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5cu2.cif.gz 5cu2.cif.gz | 302 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5cu2.ent.gz pdb5cu2.ent.gz | 245.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5cu2.json.gz 5cu2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cu/5cu2 https://data.pdbj.org/pub/pdb/validation_reports/cu/5cu2 ftp://data.pdbj.org/pub/pdb/validation_reports/cu/5cu2 ftp://data.pdbj.org/pub/pdb/validation_reports/cu/5cu2 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5ct0C  5ctpC  5cu0C  5cx9C  5mmfC  5mmrC  5mo5C  5mo6C  5mo7C  5mo8C  5modC  5moeC  5mohC  5motC  5movC  5mowC  5mp8C  5mpjC  3warS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 41467.793 Da / Num. of mol.: 2 Fragment: residues 2-329 and N-terminal extension GSMDIEFDDDADDDGSGSGSGSGS Mutation: R21S Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CSNK2A1, CK2A1 / Plasmid: pHAT4 / Production host: Homo sapiens (human) / Gene: CSNK2A1, CK2A1 / Plasmid: pHAT4 / Production host:  References: UniProt: P68400, non-specific serine/threonine protein kinase |
|---|
-Non-polymers , 5 types, 342 molecules 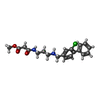








| #2: Chemical | ChemComp-551 / #3: Chemical | #4: Chemical | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.23 Å3/Da / Density % sol: 44.8 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 107mM Mes pH 6.5, 29% glycerol ethoxylate, 1 M ammonium acetate |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I02 / Wavelength: 0.9795 Å / Beamline: I02 / Wavelength: 0.9795 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Feb 16, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 1.705→167.18 Å / Num. obs: 73934 / % possible obs: 90.8 % / Redundancy: 6.4 % / Biso Wilson estimate: 28.15 Å2 / CC1/2: 0.997 / Rmerge(I) obs: 0.069 / Rpim(I) all: 0.03 / Rsym value: 0.069 / Net I/σ(I): 15.9 / Num. measured all: 473805 |
| Reflection shell | Resolution: 1.705→1.71 Å / Redundancy: 5.8 % / Rmerge(I) obs: 0.759 / Mean I/σ(I) obs: 2.1 / Num. measured all: 39589 / Num. unique all: 6828 / CC1/2: 0.817 / Rpim(I) all: 0.277 / Rsym value: 0.759 / Rejects: 0 / % possible all: 52.1 |
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3WAR Resolution: 1.705→167.18 Å / Cor.coef. Fo:Fc: 0.9475 / Cor.coef. Fo:Fc free: 0.9395 / SU R Cruickshank DPI: 0.112 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.112 / SU Rfree Blow DPI: 0.102 / SU Rfree Cruickshank DPI: 0.102
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 147.02 Å2 / Biso mean: 39.14 Å2 / Biso min: 9.35 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.23 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.705→167.18 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.7→1.74 Å / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj








