+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4yfi | ||||||
|---|---|---|---|---|---|---|---|
| Title | TNNI3K complexed with inhibitor 1 | ||||||
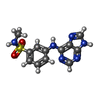
 Components Components | Serine/threonine-protein kinase TNNI3K | ||||||
 Keywords Keywords | Transferase/Transferase Inhibitor / kinase / Transferase-Transferase Inhibitor complex | ||||||
| Function / homology |  Function and homology information Function and homology informationbundle of His cell to Purkinje myocyte communication / peptidyl-serine autophosphorylation / regulation of cardiac conduction / regulation of cardiac muscle contraction / regulation of heart rate / protein phosphorylation / protein kinase activity / non-specific serine/threonine protein kinase / protein serine kinase activity / protein serine/threonine kinase activity ...bundle of His cell to Purkinje myocyte communication / peptidyl-serine autophosphorylation / regulation of cardiac conduction / regulation of cardiac muscle contraction / regulation of heart rate / protein phosphorylation / protein kinase activity / non-specific serine/threonine protein kinase / protein serine kinase activity / protein serine/threonine kinase activity / ATP binding / metal ion binding / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.7 Å X-RAY DIFFRACTION / Resolution: 2.7 Å | ||||||
 Authors Authors | Shewchuk, L.M. / Wang, L. / Lawhorn, B.G. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2015 Journal: J.Med.Chem. / Year: 2015Title: Identification of Purines and 7-Deazapurines as Potent and Selective Type I Inhibitors of Troponin I-Interacting Kinase (TNNI3K). Authors: Lawhorn, B.G. / Philp, J. / Zhao, Y. / Louer, C. / Hammond, M. / Cheung, M. / Fries, H. / Graves, A.P. / Shewchuk, L. / Wang, L. / Cottom, J.E. / Qi, H. / Zhao, H. / Totoritis, R. / Zhang, G. ...Authors: Lawhorn, B.G. / Philp, J. / Zhao, Y. / Louer, C. / Hammond, M. / Cheung, M. / Fries, H. / Graves, A.P. / Shewchuk, L. / Wang, L. / Cottom, J.E. / Qi, H. / Zhao, H. / Totoritis, R. / Zhang, G. / Schwartz, B. / Li, H. / Sweitzer, S. / Holt, D.A. / Gatto, G.J. / Kallander, L.S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4yfi.cif.gz 4yfi.cif.gz | 424.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4yfi.ent.gz pdb4yfi.ent.gz | 349 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4yfi.json.gz 4yfi.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yf/4yfi https://data.pdbj.org/pub/pdb/validation_reports/yf/4yfi ftp://data.pdbj.org/pub/pdb/validation_reports/yf/4yfi ftp://data.pdbj.org/pub/pdb/validation_reports/yf/4yfi | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
NCS ensembles :
NCS oper:
|
- Components
Components
| #1: Protein | Mass: 37267.977 Da / Num. of mol.: 4 / Fragment: UNP RESIDUES 503-831 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TNNI3K, CARK / Plasmid: pFASTBAC / Production host: Homo sapiens (human) / Gene: TNNI3K, CARK / Plasmid: pFASTBAC / Production host:  unidentified baculovirus unidentified baculovirusReferences: UniProt: Q59H18, non-specific serine/threonine protein kinase #2: Chemical | ChemComp-4CW / #3: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.86 Å3/Da / Density % sol: 57.04 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 7 / Details: 0.3 M NaK tartrate, 0.1M HEPES pH7 |
-Data collection
| Diffraction | Mean temperature: 93 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.54 Å ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.54 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: RIGAKU SATURN 944+ / Detector: CCD / Date: Jul 30, 2008 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1.54 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.6→50 Å / Num. obs: 50917 / % possible obs: 97.2 % / Redundancy: 3.7 % / Rmerge(I) obs: 0.075 / Χ2: 2.6 / Net I/av σ(I): 27.241 / Net I/σ(I): 18.7 / Num. measured all: 185934 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: _
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.7→37.82 Å / Cor.coef. Fo:Fc: 0.949 / Cor.coef. Fo:Fc free: 0.926 / SU B: 22.659 / SU ML: 0.241 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.602 / ESU R Free: 0.324 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : WITH TLS ADDED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 121.99 Å2 / Biso mean: 48.295 Å2 / Biso min: 7.45 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.7→37.82 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.7→2.77 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj