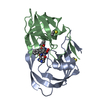
Entry Database : PDB / ID : 4xynTitle X-ray structure of Ca(2+)-S100B with human RAGE-derived W61 peptide Protein S100-B Receptor for advanced glycation endproducts-derived peptide (W61) Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)synthetic construct (others) Method / / / Resolution : 2.55 Å Authors Jensen, J.L. / Indurthi, V.S.K. / Neau, D. / Vetter, S.W. / Colbert, C.L. Journal : Acta Crystallogr.,Sect.D / Year : 2015Title : Structural insights into the binding of the human receptor for advanced glycation end products (RAGE) by S100B, as revealed by an S100B-RAGE-derived peptide complex.Authors : Jensen, J.L. / Indurthi, V.S. / Neau, D.B. / Vetter, S.W. / Colbert, C.L. History Deposition Feb 2, 2015 Deposition site / Processing site Supersession May 13, 2015 ID 4N6I Revision 1.0 May 13, 2015 Provider / Type Revision 1.1 May 20, 2015 Group Revision 1.2 May 27, 2015 Group Revision 1.3 Nov 22, 2017 Group Advisory / Derived calculations ... Advisory / Derived calculations / Refinement description / Source and taxonomy Category entity_src_gen / pdbx_entity_src_syn ... entity_src_gen / pdbx_entity_src_syn / pdbx_struct_oper_list / pdbx_unobs_or_zero_occ_atoms / pdbx_validate_close_contact / software Item _entity_src_gen.pdbx_alt_source_flag / _pdbx_entity_src_syn.pdbx_alt_source_flag ... _entity_src_gen.pdbx_alt_source_flag / _pdbx_entity_src_syn.pdbx_alt_source_flag / _pdbx_struct_oper_list.symmetry_operation / _software.classification / _software.name Revision 1.4 Feb 28, 2024 Group Advisory / Data collection ... Advisory / Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_unobs_or_zero_occ_atoms / software / struct_conn Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _software.name / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.55 Å
MOLECULAR REPLACEMENT / Resolution: 2.55 Å  Authors
Authors Citation
Citation Journal: Acta Crystallogr.,Sect.D / Year: 2015
Journal: Acta Crystallogr.,Sect.D / Year: 2015 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4xyn.cif.gz
4xyn.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4xyn.ent.gz
pdb4xyn.ent.gz PDB format
PDB format 4xyn.json.gz
4xyn.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/xy/4xyn
https://data.pdbj.org/pub/pdb/validation_reports/xy/4xyn ftp://data.pdbj.org/pub/pdb/validation_reports/xy/4xyn
ftp://data.pdbj.org/pub/pdb/validation_reports/xy/4xyn Links
Links Assembly
Assembly


 Components
Components Homo sapiens (human) / Gene: S100B / Production host:
Homo sapiens (human) / Gene: S100B / Production host: 
 X-RAY DIFFRACTION
X-RAY DIFFRACTION Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 24-ID-C / Wavelength: 0.9792 Å
/ Beamline: 24-ID-C / Wavelength: 0.9792 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 2.55→53.882 Å / SU ML: 0.41 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 27.87 / Stereochemistry target values: ML
MOLECULAR REPLACEMENT / Resolution: 2.55→53.882 Å / SU ML: 0.41 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 27.87 / Stereochemistry target values: ML Movie
Movie Controller
Controller











 PDBj
PDBj















