[English] 日本語
 Yorodumi
Yorodumi- PDB-4qoz: Crystal structure of the histone mRNA stem-loop, stem-loop bindin... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4qoz | ||||||
|---|---|---|---|---|---|---|---|
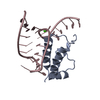
| Title | Crystal structure of the histone mRNA stem-loop, stem-loop binding protein (phosphorylated), and 3'hExo ternary complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | RNA/HYDROLASE/RNA BINDING PROTEIN / histone mRNA 3'-end processing / histone mRNA translation / microRNA homeostasis / 5.8S rRNA 3'-end maturation / ZFP100 / Lsm11 / phosphorylation / nucleus / RNA-HYDROLASE-RNA BINDING PROTEIN complex | ||||||
| Function / homology |  Function and homology information Function and homology informationhistone mRNA stem-loop binding complex / histone pre-mRNA stem-loop binding / rRNA 3'-end processing / exoribonuclease II / mRNA 3'-end processing by stem-loop binding and cleavage / cap-dependent translational initiation / exonucleolytic trimming to generate mature 3'-end of 5.8S rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / histone pre-mRNA DCP binding / histone pre-mRNA 3'end processing complex / SLBP Dependent Processing of Replication-Dependent Histone Pre-mRNAs ...histone mRNA stem-loop binding complex / histone pre-mRNA stem-loop binding / rRNA 3'-end processing / exoribonuclease II / mRNA 3'-end processing by stem-loop binding and cleavage / cap-dependent translational initiation / exonucleolytic trimming to generate mature 3'-end of 5.8S rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / histone pre-mRNA DCP binding / histone pre-mRNA 3'end processing complex / SLBP Dependent Processing of Replication-Dependent Histone Pre-mRNAs / Transport of the SLBP Dependant Mature mRNA / regulatory ncRNA-mediated gene silencing / RNA Polymerase II Transcription Termination / maturation of 5.8S rRNA / mRNA transport / Major pathway of rRNA processing in the nucleolus and cytosol / 3'-5' exonuclease activity / ribosome binding / 3'-5'-RNA exonuclease activity / rRNA binding / ribonucleoprotein complex / mRNA binding / nucleolus / RNA binding / nucleoplasm / metal ion binding / identical protein binding / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.304 Å MOLECULAR REPLACEMENT / Resolution: 2.304 Å | ||||||
 Authors Authors | Tan, D. / Tong, L. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2014 Journal: Proc.Natl.Acad.Sci.USA / Year: 2014Title: Molecular mechanisms for the regulation of histone mRNA stem-loop-binding protein by phosphorylation. Authors: Zhang, J. / Tan, D. / DeRose, E.F. / Perera, L. / Dominski, Z. / Marzluff, W.F. / Tong, L. / Hall, T.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4qoz.cif.gz 4qoz.cif.gz | 172.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4qoz.ent.gz pdb4qoz.ent.gz | 132.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4qoz.json.gz 4qoz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4qoz_validation.pdf.gz 4qoz_validation.pdf.gz | 474.8 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4qoz_full_validation.pdf.gz 4qoz_full_validation.pdf.gz | 479.2 KB | Display | |
| Data in XML |  4qoz_validation.xml.gz 4qoz_validation.xml.gz | 32.6 KB | Display | |
| Data in CIF |  4qoz_validation.cif.gz 4qoz_validation.cif.gz | 44.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qo/4qoz https://data.pdbj.org/pub/pdb/validation_reports/qo/4qoz ftp://data.pdbj.org/pub/pdb/validation_reports/qo/4qoz ftp://data.pdbj.org/pub/pdb/validation_reports/qo/4qoz | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: RNA chain | Mass: 8222.953 Da / Num. of mol.: 2 / Source method: obtained synthetically #2: Protein | Mass: 35346.699 Da / Num. of mol.: 2 Fragment: SAP domain and nuclease domain (UNP residues 55-349) Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ERI1, 3'EXO, THEX1 / Plasmid: pET24d / Production host: Homo sapiens (human) / Gene: ERI1, 3'EXO, THEX1 / Plasmid: pET24d / Production host:  References: UniProt: Q8IV48, Hydrolases; Acting on ester bonds #3: Protein | | Mass: 14152.753 Da / Num. of mol.: 1 / Fragment: RNA-binding domain (UNP residues 125-223) Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SLBP, HBP / Production host: Homo sapiens (human) / Gene: SLBP, HBP / Production host:  #4: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.39 Å3/Da / Density % sol: 48.47 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 6 Details: 8% w/v Tacsimate, pH 6.0, 18% w/v PEG3350, VAPOR DIFFUSION, HANGING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X29A / Wavelength: 1.075 Å / Beamline: X29A / Wavelength: 1.075 Å |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Jun 12, 2013 |
| Radiation | Monochromator: Rosenbaum-Rock double crystal sagittal focusing Si(111) Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.075 Å / Relative weight: 1 |
| Reflection | Resolution: 2.304→38.169 Å / Num. all: 43593 / Num. obs: 40611 / % possible obs: 93.2 % / Observed criterion σ(F): 2 / Observed criterion σ(I): 2 / Redundancy: 3.5 % / Rmerge(I) obs: 0.092 / Net I/σ(I): 9.5 |
| Reflection shell | Resolution: 2.304→2.38 Å / Redundancy: 3.5 % / Rmerge(I) obs: 0.48 / Mean I/σ(I) obs: 1.8 / % possible all: 84.5 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.304→38.169 Å / SU ML: 0.31 / σ(F): 1.33 / Phase error: 26.72 / Stereochemistry target values: ML MOLECULAR REPLACEMENT / Resolution: 2.304→38.169 Å / SU ML: 0.31 / σ(F): 1.33 / Phase error: 26.72 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.304→38.169 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj



































