[English] 日本語
 Yorodumi
Yorodumi- PDB-4n4l: Kuenenia stuttgartiensis hydroxylamine oxidoreductase soaked in h... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4n4l | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Kuenenia stuttgartiensis hydroxylamine oxidoreductase soaked in hydrazine | |||||||||
 Components Components | hydroxylamine oxidoreductase | |||||||||
 Keywords Keywords | OXIDOREDUCTASE / C-type cytochrome | |||||||||
| Function / homology |  Function and homology information Function and homology informationhydroxylamine oxidase / anaerobic respiration, using ammonium as electron donor / hydroxylamine oxidoreductase activity / anammoxosome / protein homotrimerization / nitric oxide biosynthetic process / heme binding / metal ion binding / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Candidatus Kuenenia stuttgartiensis (bacteria) Candidatus Kuenenia stuttgartiensis (bacteria) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 1.9 Å FOURIER SYNTHESIS / Resolution: 1.9 Å | |||||||||
 Authors Authors | Maalcke, W.J. / Dietl, A. / Marritt, S.J. / Butt, J.N. / Jetten, M.S.M. / Keltjens, J.T. / Barends, T.R.M.B. / Kartal, B. | |||||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2014 Journal: J.Biol.Chem. / Year: 2014Title: Structural Basis of Biological NO Generation by Octaheme Oxidoreductases. Authors: Maalcke, W.J. / Dietl, A. / Marritt, S.J. / Butt, J.N. / Jetten, M.S. / Keltjens, J.T. / Barends, T.R. / Kartal, B. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4n4l.cif.gz 4n4l.cif.gz | 137.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4n4l.ent.gz pdb4n4l.ent.gz | 104.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4n4l.json.gz 4n4l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/n4/4n4l https://data.pdbj.org/pub/pdb/validation_reports/n4/4n4l ftp://data.pdbj.org/pub/pdb/validation_reports/n4/4n4l ftp://data.pdbj.org/pub/pdb/validation_reports/n4/4n4l | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4n4jSC  4n4kC  4n4mC  4n4nC  4n4oC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 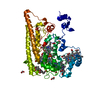
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
| ||||||||||||
| Details | Covalent trimer confirmed by SDS-PAGE, analytical ultracentrifugation, and gel filtration |
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 56543.637 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  Candidatus Kuenenia stuttgartiensis (bacteria) Candidatus Kuenenia stuttgartiensis (bacteria)References: UniProt: Q1PX48, hydroxylamine oxidase |
|---|
-Non-polymers , 6 types, 438 molecules 

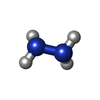

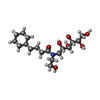






| #2: Chemical | ChemComp-PO4 / #3: Chemical | ChemComp-EDO / #4: Chemical | ChemComp-HZN / | #5: Chemical | ChemComp-HEC / #6: Chemical | ChemComp-HG1 / | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.03 Å3/Da / Density % sol: 59.46 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 7.4 Details: 1.3 M ammonium sulfate, 0.05-0.10 M sodium phosphate, pH 7.4, detergent additive cyclohexylbutanoyl-N-hydroxyethylglucamide (C-HEGA-10), VAPOR DIFFUSION, HANGING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X10SA / Wavelength: 0.9785 Å / Beamline: X10SA / Wavelength: 0.9785 Å |
| Detector | Type: PSI PILATUS 6M / Detector: PIXEL / Date: Aug 1, 2011 |
| Radiation | Monochromator: double crystal Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9785 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→46 Å / Num. all: 54927 / Num. obs: 54927 / % possible obs: 100 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Rmerge(I) obs: 0.1 / Net I/σ(I): 18 |
| Reflection shell | Resolution: 1.9→2 Å / Rmerge(I) obs: 0.464 / % possible all: 99.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: PDB ENTRY 4N4J Resolution: 1.9→45.96 Å / Cor.coef. Fo:Fc: 0.964 / Cor.coef. Fo:Fc free: 0.958 / SU B: 1.832 / SU ML: 0.055 / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.104 / ESU R Free: 0.093 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 14.714 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→45.96 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj














