+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4fub | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of the Urokinase | ||||||
 Components Components | Urokinase-type plasminogen activator | ||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / HYDROLASE / HYDROLASE-HYDROLASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationu-plasminogen activator / regulation of smooth muscle cell-matrix adhesion / regulation of integrin-mediated signaling pathway / urokinase plasminogen activator signaling pathway / regulation of plasminogen activation / regulation of fibrinolysis / protein complex involved in cell-matrix adhesion / regulation of wound healing / negative regulation of plasminogen activation / serine-type endopeptidase complex ...u-plasminogen activator / regulation of smooth muscle cell-matrix adhesion / regulation of integrin-mediated signaling pathway / urokinase plasminogen activator signaling pathway / regulation of plasminogen activation / regulation of fibrinolysis / protein complex involved in cell-matrix adhesion / regulation of wound healing / negative regulation of plasminogen activation / serine-type endopeptidase complex / regulation of smooth muscle cell migration / Dissolution of Fibrin Clot / smooth muscle cell migration / plasminogen activation / regulation of cell adhesion mediated by integrin / tertiary granule membrane / negative regulation of fibrinolysis / regulation of cell adhesion / positive regulation of epidermal growth factor receptor signaling pathway / serine protease inhibitor complex / specific granule membrane / fibrinolysis / chemotaxis / blood coagulation / regulation of cell population proliferation / response to hypoxia / positive regulation of cell migration / receptor ligand activity / serine-type endopeptidase activity / external side of plasma membrane / focal adhesion / Neutrophil degranulation / cell surface / signal transduction / proteolysis / extracellular space / extracellular exosome / extracellular region / plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å | ||||||
 Authors Authors | Kang, Y.N. / Stuckey, J.A. / Nienaber, V. / Giranda, V. | ||||||
 Citation Citation |  Journal: to be published Journal: to be publishedTitle: Crystal Structure of the Urokinase Authors: Kang, Y.N. / Stuckey, J.A. / Nienaber, V. / Giranda, V. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4fub.cif.gz 4fub.cif.gz | 120.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4fub.ent.gz pdb4fub.ent.gz | 88.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4fub.json.gz 4fub.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fu/4fub https://data.pdbj.org/pub/pdb/validation_reports/fu/4fub ftp://data.pdbj.org/pub/pdb/validation_reports/fu/4fub ftp://data.pdbj.org/pub/pdb/validation_reports/fu/4fub | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4fu7C  4fu8C  4fu9C  4fucC  4fudC  4fueC  4fufC  4fugC  4fuhC  4fuiC  4fujC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 27715.600 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PLAU / Production host: Homo sapiens (human) / Gene: PLAU / Production host:  |
|---|
-Non-polymers , 7 types, 259 molecules 

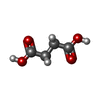









| #2: Chemical | ChemComp-4UP / | ||||||
|---|---|---|---|---|---|---|---|
| #3: Chemical | ChemComp-SO4 / | ||||||
| #4: Chemical | ChemComp-SIN / | ||||||
| #5: Chemical | ChemComp-GOL / #6: Chemical | ChemComp-15P / | #7: Chemical | ChemComp-ACT / #8: Water | ChemComp-HOH / | |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.09 Å3/Da / Density % sol: 41.15 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop Details: 0.15 M Li2SO4, 20% polyethylene glycol MW 4000 in succinate buffer, pH 4.8-6.0, VAPOR DIFFUSION, HANGING DROP, temperature 298.0K PH range: 4.8-6.0 |
-Data collection
| Diffraction | Mean temperature: 160 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU / Wavelength: 1.5418 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: RIGAKU RAXIS II / Detector: IMAGE PLATE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.6→40 Å / % possible obs: 86.3 % / Rmerge(I) obs: 0.122 / Χ2: 1.259 / Net I/σ(I): 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 1.9→17.74 Å / Cor.coef. Fo:Fc: 0.9521 / Cor.coef. Fo:Fc free: 0.9409 / Occupancy max: 1 / Occupancy min: 0.5 / SU R Cruickshank DPI: 0.156 / Cross valid method: THROUGHOUT / σ(F): 0 MOLECULAR REPLACEMENT / Resolution: 1.9→17.74 Å / Cor.coef. Fo:Fc: 0.9521 / Cor.coef. Fo:Fc free: 0.9409 / Occupancy max: 1 / Occupancy min: 0.5 / SU R Cruickshank DPI: 0.156 / Cross valid method: THROUGHOUT / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 70.11 Å2 / Biso mean: 18.5997 Å2 / Biso min: 6.14 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.164 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→17.74 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.9→2.02 Å / Total num. of bins used: 9
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -13.7272 Å / Origin y: -9.4447 Å / Origin z: 10.9941 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: { A|1 - A|246 } |
 Movie
Movie Controller
Controller













 PDBj
PDBj







