[English] 日本語
 Yorodumi
Yorodumi- PDB-3wh1: Crystal Structure of a Family GH19 Chitinase from Bryum coronatum... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3wh1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of a Family GH19 Chitinase from Bryum coronatum in complex with (GlcNAc)4 at 1.0 A resolution | |||||||||
 Components Components | Chitinase A | |||||||||
 Keywords Keywords | HYDROLASE / Chitinase / Carbohydrate | |||||||||
| Function / homology |  Function and homology information Function and homology informationchitinase activity / chitin catabolic process / defense response to fungus / cell wall macromolecule catabolic process / carbohydrate metabolic process Similarity search - Function | |||||||||
| Biological species |  Bryum coronatum (plant) Bryum coronatum (plant) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1 Å MOLECULAR REPLACEMENT / Resolution: 1 Å | |||||||||
 Authors Authors | Numata, T. / Umemoto, N. / Ohnuma, T. / Fukamizo, T. | |||||||||
 Citation Citation |  Journal: Biochim.Biophys.Acta / Year: 2014 Journal: Biochim.Biophys.Acta / Year: 2014Title: Crystal structure of a "loopless" GH19 chitinase in complex with chitin tetrasaccharide spanning the catalytic center. Authors: Ohnuma, T. / Umemoto, N. / Nagata, T. / Shinya, S. / Numata, T. / Taira, T. / Fukamizo, T. | |||||||||
| History |
|
- Structure visualization
Structure visualization
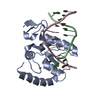
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3wh1.cif.gz 3wh1.cif.gz | 101.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3wh1.ent.gz pdb3wh1.ent.gz | 75.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3wh1.json.gz 3wh1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  3wh1_validation.pdf.gz 3wh1_validation.pdf.gz | 775.8 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  3wh1_full_validation.pdf.gz 3wh1_full_validation.pdf.gz | 775.7 KB | Display | |
| Data in XML |  3wh1_validation.xml.gz 3wh1_validation.xml.gz | 11.8 KB | Display | |
| Data in CIF |  3wh1_validation.cif.gz 3wh1_validation.cif.gz | 17.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wh/3wh1 https://data.pdbj.org/pub/pdb/validation_reports/wh/3wh1 ftp://data.pdbj.org/pub/pdb/validation_reports/wh/3wh1 ftp://data.pdbj.org/pub/pdb/validation_reports/wh/3wh1 | HTTPS FTP |
-Related structure data
| Related structure data |  4ij4SC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 22738.990 Da / Num. of mol.: 1 / Fragment: UNP residues 24-228 / Mutation: E61A Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Bryum coronatum (plant) / Gene: bcchiA / Plasmid: pET-22b / Production host: Bryum coronatum (plant) / Gene: bcchiA / Plasmid: pET-22b / Production host:  | ||||
|---|---|---|---|---|---|
| #2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source | ||||
| #3: Chemical | ChemComp-EDO / #4: Water | ChemComp-HOH / | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.07 Å3/Da / Density % sol: 40.71 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: 0.09M Tris-HCl(pH 8.5), 1.35M di-Ammonium hydrogen phosphate, 0.001M CoCl2, 0.01M Sodium acetate(pH 4.6), 0.1M 1,6-Hexanediol, VAPOR DIFFUSION, SITTING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 95 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Photon Factory Photon Factory  / Beamline: BL-17A / Wavelength: 0.98 Å / Beamline: BL-17A / Wavelength: 0.98 Å |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Oct 23, 2010 |
| Radiation | Monochromator: Numerical link type Si(111) double crystal monochromater, liquid nitrogen cooling Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.98 Å / Relative weight: 1 |
| Reflection | Resolution: 1→50 Å / Num. obs: 98800 / % possible obs: 98.6 % / Redundancy: 6.3 % / Rmerge(I) obs: 0.086 / Net I/σ(I): 33.25 |
| Reflection shell | Resolution: 1→1.04 Å / Redundancy: 3.3 % / Rmerge(I) obs: 0.319 / Mean I/σ(I) obs: 4.13 / Num. unique all: 30901 / % possible all: 94.2 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4IJ4 Resolution: 1→44.06 Å / Cor.coef. Fo:Fc: 0.966 / Cor.coef. Fo:Fc free: 0.963 / SU B: 0.473 / SU ML: 0.012 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / ESU R: 0.024 / ESU R Free: 0.023 / Stereochemistry target values: MAXIMUM LIKELIHOOD
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 7.163 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1→44.06 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 0.999→1.025 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller











 PDBj
PDBj


