[English] 日本語
 Yorodumi
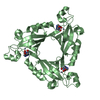
Yorodumi- PDB-3i2b: The crystal structure of human 6 Pyruvoyl Tetrahydrobiopterin Synthase -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3i2b | ||||||
|---|---|---|---|---|---|---|---|
| Title | The crystal structure of human 6 Pyruvoyl Tetrahydrobiopterin Synthase | ||||||
 Components Components | 6-pyruvoyl tetrahydrobiopterin synthase | ||||||
 Keywords Keywords | LYASE / 6 pyruvoyl tetrahydrobiopterin synthase / PTS / PTP synthase / Structural Genomics / Structural Genomics Consortium / SGC / Disease mutation / Metal-binding / Phenylketonuria / Phosphoprotein / Tetrahydrobiopterin biosynthesis | ||||||
| Function / homology |  Function and homology information Function and homology information6-pyruvoyltetrahydropterin synthase / 6-pyruvoyltetrahydropterin synthase activity / tetrahydrobiopterin biosynthetic process / amino acid metabolic process / Tetrahydrobiopterin (BH4) synthesis, recycling, salvage and regulation / central nervous system development / mitochondrion / metal ion binding / identical protein binding / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Ugochukwu, E. / Cocking, R. / Pilka, E. / Yue, W.W. / Bray, J.E. / Chaikuad, A. / Krojer, T. / Muniz, J. / von Delft, F. / Bountra, C. ...Ugochukwu, E. / Cocking, R. / Pilka, E. / Yue, W.W. / Bray, J.E. / Chaikuad, A. / Krojer, T. / Muniz, J. / von Delft, F. / Bountra, C. / Arrowsmith, C.H. / Weigelt, J. / Edwards, A. / Oppermann, U. / Structural Genomics Consortium (SGC) | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: The crystal structure of human 6 Pyruvoyl Tetrahydrobiopterin Synthase Authors: Ugochukwu, E. / Cocking, R. / Pilka, E. / Yue, W.W. / Bray, J.E. / Chaikuad, A. / Krojer, T. / Muniz, J. / Oppermann, U. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3i2b.cif.gz 3i2b.cif.gz | 329.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3i2b.ent.gz pdb3i2b.ent.gz | 266.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3i2b.json.gz 3i2b.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/i2/3i2b https://data.pdbj.org/pub/pdb/validation_reports/i2/3i2b ftp://data.pdbj.org/pub/pdb/validation_reports/i2/3i2b ftp://data.pdbj.org/pub/pdb/validation_reports/i2/3i2b | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1gtqS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
|
 Movie
Movie Controller
Controller











 PDBj
PDBj



