[English] 日本語
 Yorodumi
Yorodumi- PDB-2rs5: STRUCTURAL ANALYSIS OF ANTIVIRAL AGENTS THAT INTERACT WITH THE CA... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2rs5 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | STRUCTURAL ANALYSIS OF ANTIVIRAL AGENTS THAT INTERACT WITH THE CAPSID OF HUMAN RHINOVIRUSES | ||||||||||||
 Components Components | (HUMAN RHINOVIRUS 14 COAT PROTEIN (SUBUNIT ...) x 4 | ||||||||||||
 Keywords Keywords | VIRUS / RHINOVIRUS COAT PROTEIN / Icosahedral virus | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationlysis of host organelle involved in viral entry into host cell / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity ...lysis of host organelle involved in viral entry into host cell / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / ATP hydrolysis activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | ||||||||||||
| Biological species |  Human rhinovirus 14 Human rhinovirus 14 | ||||||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 3 Å X-RAY DIFFRACTION / Resolution: 3 Å | ||||||||||||
 Authors Authors | Badger, J. / Smith, T.J. / Rossmann, M.G. | ||||||||||||
 Citation Citation |  Journal: Proteins / Year: 1989 Journal: Proteins / Year: 1989Title: Structural analysis of antiviral agents that interact with the capsid of human rhinoviruses. Authors: Badger, J. / Minor, I. / Oliveira, M.A. / Smith, T.J. / Rossmann, M.G. #1:  Journal: To be Published Journal: To be PublishedTitle: The Use of Molecular Replacement Phases for the Refinement of the Human Rhinovirus 14 Structure Authors: Arnold, E. / Rossmann, M.G. #2:  Journal: To be Published Journal: To be PublishedTitle: Analysis of the Structure of a Common Cold Virus, Human Rhinovirus 14, Refined at a Resolution of 3.0 Angstroms Authors: Arnold, E. / Rossmann, M.G. #3:  Journal: J.Mol.Biol. / Year: 1989 Journal: J.Mol.Biol. / Year: 1989Title: Three-Dimensional Structures of Drug-Resistant Mutants of Human Rhinovirus 14 Authors: Badger, J. / Krishnaswamy, S. / Kremer, M.J. / Oliveira, M.A. / Rossmann, M.G. / Heinz, B.A. / Rueckert, R.R. / Dutko, F.J. / Mckinlay, M.A. #4:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1988 Journal: Proc.Natl.Acad.Sci.USA / Year: 1988Title: Structural Analysis of a Series of Antiviral Agents Complexed with Human Rhinovirus 14 Authors: Badger, J. / Minor, I. / Kremer, M.J. / Oliveira, M.A. / Smith, T.J. / Griffith, J.P. / Guerin, D.M.A. / Krishnaswamy, S. / Luo, M. / Rossmann, M.G. / Mckinlay, M.A. / Diana, G.D. / Dutko, F. ...Authors: Badger, J. / Minor, I. / Kremer, M.J. / Oliveira, M.A. / Smith, T.J. / Griffith, J.P. / Guerin, D.M.A. / Krishnaswamy, S. / Luo, M. / Rossmann, M.G. / Mckinlay, M.A. / Diana, G.D. / Dutko, F.J. / Fancher, M. / Rueckert, R.R. / Heinz, B.A. #5:  Journal: Acta Crystallogr.,Sect.A / Year: 1987 Journal: Acta Crystallogr.,Sect.A / Year: 1987Title: The Structure Determination of a Common Cold Virus, Human Rhinovirus 14 Authors: Arnold, E. / Vriend, G. / Luo, M. / Griffith, J.P. / Kamer, G. / Erickson, J.W. / Johnson, J.E. / Rossmann, M.G. #6:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1987 Journal: Proc.Natl.Acad.Sci.USA / Year: 1987Title: Implications of the Picornavirus Capsid Structure for Polyprotein Structure Authors: Arnold, E. / Luo, M. / Vriend, G. / Rossmann, M.G. / Palmenberg, A.C. / Parks, G.D. / Nicklin, M.J.H. / Wimmer, E. #7:  Journal: Science / Year: 1986 Journal: Science / Year: 1986Title: The Site of Attachment in Human Rhinovirus 14 for Antiviral Agents that Inhibit Uncoating Authors: Smith, T.J. / Kremer, M.J. / Luo, M. / Vriend, G. / Arnold, E. / Kamer, G. / Rossmann, M.G. / Mckinlay, M.A. / Diana, G.D. / Otto, M.J. #8:  Journal: Chem.Scr. / Year: 1987 Journal: Chem.Scr. / Year: 1987Title: The Structure of a Human Common Cold Virus (Rhinovirus 14) and its Evolutionary Relations to Other Viruses Authors: Rossmann, M.G. / Arnold, E. / Erickson, J.W. / Frankenberger, E.A. / Griffith, J.P. / Hecht, H.-J. / Johnson, J.E. / Kamer, G. / Luo, M. / Vriend, G. #9:  Journal: Nature / Year: 1985 Journal: Nature / Year: 1985Title: Structure of a Human Common Cold Virus and Functional Relationship to Other Picornaviruses Authors: Rossmann, M.G. / Arnold, E. / Erickson, J.W. / Frankenberger, E.A. / Griffith, J.P. / Hecht, H.-J. / Johnson, J.E. / Kamer, G. / Luo, M. / Mosser, A.G. / Rueckert, R.R. / Sherry, B. / Vriend, G. #10:  Journal: J.Mol.Biol. / Year: 1984 Journal: J.Mol.Biol. / Year: 1984Title: Virion Orientation in Cubic Crystals of the Human Common Cold Virus Hrv14 Authors: Arnold, E. / Erickson, J.W. / Fout, G.S. / Frankenberger, E.A. / Hecht, H.-J. / Luo, M. / Rossmann, M.G. / Rueckert, R.R. #11:  Journal: J.Mol.Biol. / Year: 1984 Journal: J.Mol.Biol. / Year: 1984Title: Picornaviruses of Two Different Genera Have Similar Structures Authors: Luo, M. / Arnold, E. / Erickson, J.W. / Rossmann, M.G. / Boege, U. / Scraba, D.G. #12:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1983 Journal: Proc.Natl.Acad.Sci.USA / Year: 1983Title: Crystallization of a Common Cold Virus, Human Rhinovirus 14. (Quote)Isomorphism(Quote) with Poliovirus Crystals Authors: Erickson, J.W. / Frankenberger, E.A. / Rossmann, M.G. / Fout, G.S. / Medappa, K.C. / Rueckert, R.R. | ||||||||||||
| History |
| ||||||||||||
| Remark 700 | SHEET THERE IS A BIFURCATED SHEET IN EACH OF CHAINS *1*, *2*, AND *3*. TO REPRESENT THIS FEATURE ...SHEET THERE IS A BIFURCATED SHEET IN EACH OF CHAINS *1*, *2*, AND *3*. TO REPRESENT THIS FEATURE REDUNDANT SHEETS ARE DEFINED. THUS SHEETS B11 AND B12 DIFFER ONLY IN STRAND 4, SHEETS B12 AND B22 DIFFER ONLY IN STRAND 4, AND SHEETS B13 AND B23 DIFFER IN STRANDS 1 AND 4 (DOUBLY BIFURCATED). |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2rs5.cif.gz 2rs5.cif.gz | 156.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2rs5.ent.gz pdb2rs5.ent.gz | 117.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2rs5.json.gz 2rs5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rs/2rs5 https://data.pdbj.org/pub/pdb/validation_reports/rs/2rs5 ftp://data.pdbj.org/pub/pdb/validation_reports/rs/2rs5 ftp://data.pdbj.org/pub/pdb/validation_reports/rs/2rs5 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1r08C  2r04C  2r06C  2r07C  2rm2C  2rr1C  2rs1C  2rs3C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | x 60
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | x 5
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | x 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | x 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atom site foot note | 1: RESIDUE PRO 2 83 IS A CIS PROLINE. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: (Hermann–Mauguin notation: 532 / Schoenflies symbol: I (icosahedral)) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
-HUMAN RHINOVIRUS 14 COAT PROTEIN (SUBUNIT ... , 4 types, 4 molecules 1234
| #1: Protein | Mass: 32560.549 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human rhinovirus 14 / Genus: Rhinovirus / Species: Human rhinovirus B / Cell line (production host): HeLa cells / Production host: Human rhinovirus 14 / Genus: Rhinovirus / Species: Human rhinovirus B / Cell line (production host): HeLa cells / Production host:  Homo sapiens (human) / References: UniProt: P03303 Homo sapiens (human) / References: UniProt: P03303 |
|---|---|
| #2: Protein | Mass: 28501.361 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human rhinovirus 14 / Genus: Rhinovirus / Species: Human rhinovirus B / Cell line (production host): HeLa cells / Production host: Human rhinovirus 14 / Genus: Rhinovirus / Species: Human rhinovirus B / Cell line (production host): HeLa cells / Production host:  Homo sapiens (human) / References: UniProt: P03303 Homo sapiens (human) / References: UniProt: P03303 |
| #3: Protein | Mass: 26236.754 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human rhinovirus 14 / Genus: Rhinovirus / Species: Human rhinovirus B / Cell line (production host): HeLa cells / Production host: Human rhinovirus 14 / Genus: Rhinovirus / Species: Human rhinovirus B / Cell line (production host): HeLa cells / Production host:  Homo sapiens (human) / References: UniProt: P03303 Homo sapiens (human) / References: UniProt: P03303 |
| #4: Protein | Mass: 7183.863 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human rhinovirus 14 / Genus: Rhinovirus / Species: Human rhinovirus B / Cell line (production host): HeLa cells / Production host: Human rhinovirus 14 / Genus: Rhinovirus / Species: Human rhinovirus B / Cell line (production host): HeLa cells / Production host:  Homo sapiens (human) / References: UniProt: P03303 Homo sapiens (human) / References: UniProt: P03303 |
-Non-polymers , 2 types, 3 molecules 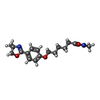


| #5: Chemical | ChemComp-W56 / |
|---|---|
| #6: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal grow | *PLUS pH: 7.2 / Method: vapor diffusion, hanging drop / Details: Arnold, E., (1984) J.Mol.Biol., 177, 417. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Components of the solutions | *PLUS
|
-Data collection
| Reflection | *PLUS Highest resolution: 3 Å / Num. obs: 180005 / Observed criterion σ(I): 3.5 / Num. measured all: 246095 / Rmerge(I) obs: 0.111 |
|---|
- Processing
Processing
| Software | Name: REAL-SPACE / Version: REFINEMENT / Classification: refinement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Highest resolution: 3 Å | ||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 3 Å
| ||||||||||||
| Refinement | *PLUS Rfactor obs: 0.136 | ||||||||||||
| Solvent computation | *PLUS | ||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller























 PDBj
PDBj


