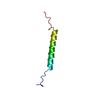
Entry Database : PDB / ID : 2llmTitle Structure of amyloid precursor protein's transmembrane domain Amyloid beta A4 protein Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / Model details fewest violations, model 1 Authors Nadezhdin, K.D. / Bocharova, O.V. / Bocharov, E.V. / Arseniev, A.S. Journal : Acta Naturae / Year : 2011Title : Structural and dynamic study of the transmembrane domain of the amyloid precursor protein.Authors : Nadezhdin, K.D. / Bocharova, O.V. / Bocharov, E.V. / Arseniev, A.S. History Deposition Nov 15, 2011 Deposition site / Processing site Revision 1.0 Jun 20, 2012 Provider / Type Revision 1.1 Jun 14, 2023 Group / Database references / OtherCategory database_2 / pdbx_database_status ... database_2 / pdbx_database_status / pdbx_nmr_software / pdbx_nmr_spectrometer / struct_ref_seq_dif Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_nmr_data / _pdbx_nmr_software.name / _pdbx_nmr_spectrometer.model / _struct_ref_seq_dif.details Revision 1.2 May 15, 2024 Group / Database references / Category / chem_comp_bond / database_2 / Item
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) Authors
Authors Citation
Citation Journal: Acta Naturae / Year: 2011
Journal: Acta Naturae / Year: 2011 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2llm.cif.gz
2llm.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2llm.ent.gz
pdb2llm.ent.gz PDB format
PDB format 2llm.json.gz
2llm.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 2llm_validation.pdf.gz
2llm_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 2llm_full_validation.pdf.gz
2llm_full_validation.pdf.gz 2llm_validation.xml.gz
2llm_validation.xml.gz 2llm_validation.cif.gz
2llm_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/ll/2llm
https://data.pdbj.org/pub/pdb/validation_reports/ll/2llm ftp://data.pdbj.org/pub/pdb/validation_reports/ll/2llm
ftp://data.pdbj.org/pub/pdb/validation_reports/ll/2llm Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: APP, A4, AD1 / Production host:
Homo sapiens (human) / Gene: APP, A4, AD1 / Production host: 
 Sample preparation
Sample preparation Processing
Processing Movie
Movie Controller
Controller








 PDBj
PDBj














 HSQC
HSQC