[English] 日本語
 Yorodumi
Yorodumi- PDB-2j8g: Crystal structure of the modular Cpl-1 endolysin complexed with a... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2j8g | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the modular Cpl-1 endolysin complexed with a peptidoglycan analogue (E94Q mutant in complex with a tetrasaccharide- pentapeptide) | |||||||||
 Components Components | LYSOZYME | |||||||||
 Keywords Keywords | HYDROLASE / ANTIMICROBIAL / MUEIN HYDROLASE / BACTERIOLYTIC ENZYME / PNEUMOCOCCAL CELL WALL DEGRADATION / LYSOZYME / GLYCOSIDASE / MULTIMODULAR | |||||||||
| Function / homology |  Function and homology information Function and homology informationviral release from host cell by cytolysis / peptidoglycan catabolic process / cell wall macromolecule catabolic process / lysozyme / lysozyme activity / defense response to bacterium Similarity search - Function | |||||||||
| Biological species |  BACTERIOPHAGE CP-1 (virus) BACTERIOPHAGE CP-1 (virus) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.69 Å MOLECULAR REPLACEMENT / Resolution: 1.69 Å | |||||||||
 Authors Authors | Perez-Dorado, I. / Hermoso, J.A. | |||||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2007 Journal: J.Biol.Chem. / Year: 2007Title: Elucidation of the Molecular Recognition of Bacterial Cell Wall by Modular Pneumococcal Phage Endolysin Cpl-1. Authors: Perez-Dorado, I. / Campillo, N.E. / Monterroso, B. / Hesek, D. / Lee, M. / Paez, J.A. / Garcia, P. / Martinez-Ripoll, M. / Garcia, J.L. / Mobashery, S. / Menendez, M. / Hermoso, J.A. #1:  Journal: Structure / Year: 2003 Journal: Structure / Year: 2003Title: Structural Basis for Selective Recognition of Pneumococcal Cell Wall by Modular Endolysin from Phage Cp-1 Authors: Hermoso, J.A. / Monterroso, B. / Albert, A. / Galan, B. / Ahrazem, O. / Garcia, P. / Martinez-Ripoll, M. / Garcia, J.L. / Menendez, M. #2: Journal: Acta Crystallogr.,Sect.D / Year: 2002 Title: Crystallization and Preliminary X-Ray Diffraction Studies of the Complete Modular Endolysin from Cp- 1, a Phage Infecting Streptococcus Pneumoniae Authors: Monterroso, B. / Albert, A. / Martinez-Ripoll, M. / Garcia, P. / Garcia, J.L. / Menendez, M. / Hermoso, J.A. | |||||||||
| History |
| |||||||||
| Remark 700 | SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AA" IN EACH CHAIN ON SHEET RECORDS BELOW ... SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 10-STRANDED BARREL THIS IS REPRESENTED BY A 11-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2j8g.cif.gz 2j8g.cif.gz | 96.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2j8g.ent.gz pdb2j8g.ent.gz | 71.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2j8g.json.gz 2j8g.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/j8/2j8g https://data.pdbj.org/pub/pdb/validation_reports/j8/2j8g ftp://data.pdbj.org/pub/pdb/validation_reports/j8/2j8g ftp://data.pdbj.org/pub/pdb/validation_reports/j8/2j8g | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2ixuC  2ixvC  2j8fC  1h09S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / Sugars , 2 types, 2 molecules A
| #1: Protein | Mass: 39220.410 Da / Num. of mol.: 1 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)  BACTERIOPHAGE CP-1 (virus) / Production host: BACTERIOPHAGE CP-1 (virus) / Production host:  |
|---|---|
| #2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-N-acetyl-beta-muramic acid-(1-4)-2-acetamido-2-deoxy- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-N-acetyl-beta-muramic acid-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
-Non-polymers , 5 types, 458 molecules 
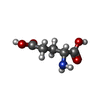







| #3: Chemical | ChemComp-ALA / |
|---|---|
| #4: Chemical | ChemComp-DGL / |
| #5: Chemical | ChemComp-LYS / |
| #6: Chemical | ChemComp-FMT / |
| #7: Water | ChemComp-HOH / |
-Details
| Compound details | ENGINEERED |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.16 Å3/Da / Density % sol: 61.02 % / Description: NONE |
|---|---|
| Crystal grow | pH: 6 / Details: pH 6.0 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 0.976 / Beamline: ID29 / Wavelength: 0.976 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Jul 14, 2006 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.976 Å / Relative weight: 1 |
| Reflection | Resolution: 1.51→63.62 Å / Num. obs: 58301 / % possible obs: 92.6 % / Observed criterion σ(I): 1 / Redundancy: 5.5 % / Rmerge(I) obs: 0.12 / Net I/σ(I): 3.3 |
| Reflection shell | Resolution: 1.69→1.81 Å / Redundancy: 3.4 % / Rmerge(I) obs: 0.5 / Mean I/σ(I) obs: 1.1 / % possible all: 71.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1H09 Resolution: 1.69→63.25 Å / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 20.707 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.69→63.25 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.69→1.73 Å / Total num. of bins used: 20 /
|
 Movie
Movie Controller
Controller













 PDBj
PDBj





