Entry Database : PDB / ID : 2i08Title Solvation effect in conformational changes of EF-hand proteins: X-ray structure of Ca2+-saturated double mutant Q41L-K75I of N-domain of calmodulin Calmodulin Keywords / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / Resolution : 2 Å Authors Ababou, A. / Parkinson, G.N. / Desjarlais, J.R. / Djordjevic, S. Journal : To be Published Title : Solvation effect in conformational changes of EF-hand proteins: X-ray structure of double mutant Q41L-K75I of N-domain of calmodulinAuthors : Ababou, A. / Parkinson, G.N. / Desjarlais, J.R. / Djordjevic, S. History Deposition Aug 10, 2006 Deposition site / Processing site Revision 1.0 Aug 21, 2007 Provider / Type Revision 1.1 Jul 13, 2011 Group / Version format complianceRevision 1.2 Oct 20, 2021 Group / Derived calculations / Category / struct_ref_seq_dif / struct_siteItem _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id Revision 1.3 Aug 30, 2023 Group / Refinement descriptionCategory / chem_comp_bond / pdbx_initial_refinement_model
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2 Å
MOLECULAR REPLACEMENT / Resolution: 2 Å  Authors
Authors Citation
Citation Journal: To be Published
Journal: To be Published Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2i08.cif.gz
2i08.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2i08.ent.gz
pdb2i08.ent.gz PDB format
PDB format 2i08.json.gz
2i08.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/i0/2i08
https://data.pdbj.org/pub/pdb/validation_reports/i0/2i08 ftp://data.pdbj.org/pub/pdb/validation_reports/i0/2i08
ftp://data.pdbj.org/pub/pdb/validation_reports/i0/2i08
 Links
Links Assembly
Assembly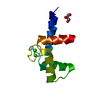
 Components
Components Homo sapiens (human) / Strain: HUMAN / Gene: calm1 / Plasmid: PET3A / Species (production host): Escherichia coli / Production host:
Homo sapiens (human) / Strain: HUMAN / Gene: calm1 / Plasmid: PET3A / Species (production host): Escherichia coli / Production host: 
 X-RAY DIFFRACTION
X-RAY DIFFRACTION Sample preparation
Sample preparation ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 Å
ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller










 PDBj
PDBj

























