[English] 日本語
 Yorodumi
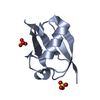
Yorodumi- PDB-2fcq: X-ray Crystal Structure of a Chemically Synthesized Ubiquitin wit... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2fcq | ||||||
|---|---|---|---|---|---|---|---|
| Title | X-ray Crystal Structure of a Chemically Synthesized Ubiquitin with a Cubic Space Group | ||||||
 Components Components | Ubiquitin | ||||||
 Keywords Keywords | STRUCTURAL PROTEIN / ubiquitin | ||||||
| Function / homology |  Function and homology information Function and homology informationPhosphatidylinositol 3-kinase Catalytic Subunit; Chain A, domain 1 / : / Ubiquitin-like (UB roll) / Ubiquitin domain signature. / Ubiquitin conserved site / Ubiquitin domain / Ubiquitin family / Ubiquitin homologues / Ubiquitin domain profile. / Ubiquitin-like domain ...Phosphatidylinositol 3-kinase Catalytic Subunit; Chain A, domain 1 / : / Ubiquitin-like (UB roll) / Ubiquitin domain signature. / Ubiquitin conserved site / Ubiquitin domain / Ubiquitin family / Ubiquitin homologues / Ubiquitin domain profile. / Ubiquitin-like domain / Ubiquitin-like domain superfamily / Roll / Alpha Beta Similarity search - Domain/homology | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.3 Å MOLECULAR REPLACEMENT / Resolution: 3.3 Å | ||||||
 Authors Authors | Bang, D. / Gribenko, A.V. / Tereshko, V. / Kossiakoff, A.A. / Kent, S.B. / Makhatadze, G.I. | ||||||
 Citation Citation |  Journal: Nat.Chem.Biol. / Year: 2006 Journal: Nat.Chem.Biol. / Year: 2006Title: Dissecting the energetics of protein alpha-helix C-cap termination through chemical protein synthesis. Authors: Bang, D. / Gribenko, A.V. / Tereshko, V. / Kossiakoff, A.A. / Kent, S.B. / Makhatadze, G.I. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2fcq.cif.gz 2fcq.cif.gz | 39.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2fcq.ent.gz pdb2fcq.ent.gz | 27.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2fcq.json.gz 2fcq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fc/2fcq https://data.pdbj.org/pub/pdb/validation_reports/fc/2fcq ftp://data.pdbj.org/pub/pdb/validation_reports/fc/2fcq ftp://data.pdbj.org/pub/pdb/validation_reports/fc/2fcq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2fcmC  2fcnC  2fcsC  1yiwS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 8558.793 Da / Num. of mol.: 2 / Fragment: residues 1-76 / Mutation: M1L / Source method: obtained synthetically Details: The protein was chemically synthesized. The sequence of the protein can be naturally found in Homo sapiens (Human) References: GenBank: 15928840, UniProt: Q9PST8*PLUS #2: Chemical | ChemComp-CD / |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.82 Å3/Da / Density % sol: 56.41 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7.75 Details: by mixing 2 ul of ubiquitin solution (20 mg/ml) and 0.5 ul of crystallization buffer solution. The crystallization buffer was prepared by mixing 3ml of HEPES buffer (0.1M), 3ml of ...Details: by mixing 2 ul of ubiquitin solution (20 mg/ml) and 0.5 ul of crystallization buffer solution. The crystallization buffer was prepared by mixing 3ml of HEPES buffer (0.1M), 3ml of poly(ethylene glycol) 3350 (25%, w/v), and 0.2ml of 1M cadmium acetate, pH 7.75, VAPOR DIFFUSION, HANGING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 17-ID / Wavelength: 1 Å / Beamline: 17-ID / Wavelength: 1 Å |
| Detector | Type: ADSC QUANTUM 210 / Detector: CCD / Date: Nov 23, 2004 / Details: MIRRORS |
| Radiation | Monochromator: SI(111) DOUBLE-CRYSTAL / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 3.3→30 Å / Num. obs: 3300 / % possible obs: 99.6 % / Redundancy: 11.9 % / Rmerge(I) obs: 0.072 / Net I/σ(I): 23.9 |
| Reflection shell | Resolution: 3.3→3.42 Å / Redundancy: 12.5 % / Rmerge(I) obs: 0.66 / Mean I/σ(I) obs: 3.2 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 1YIW, CHAIN B Resolution: 3.3→20 Å / Cor.coef. Fo:Fc: 0.926 / Cor.coef. Fo:Fc free: 0.903 / SU B: 62.771 / SU ML: 0.458 / TLS residual ADP flag: LIKELY RESIDUAL / Cross valid method: THROUGHOUT / ESU R Free: 0.624 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 85 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.3→20 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 3.301→3.384 Å / Total num. of bins used: 20 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj


