+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1xca | ||||||
|---|---|---|---|---|---|---|---|
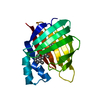
| Title | APO-CELLULAR RETINOIC ACID BINDING PROTEIN II | ||||||
 Components Components | CELLULAR RETINOIC ACID BINDING PROTEIN TYPE II | ||||||
 Keywords Keywords | RETINOIC ACID TRANSPORT / CRABPII / RETINOIC ACID / LIGAND ENTRY / VITAMIN A | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of collateral sprouting / retinoid binding / retinoic acid binding / retinal binding / embryonic forelimb morphogenesis / retinoic acid metabolic process / retinol binding / Signaling by Retinoic Acid / epidermis development / fatty acid transport ...positive regulation of collateral sprouting / retinoid binding / retinoic acid binding / retinal binding / embryonic forelimb morphogenesis / retinoic acid metabolic process / retinol binding / Signaling by Retinoic Acid / epidermis development / fatty acid transport / cyclin binding / fatty acid binding / regulation of DNA-templated transcription / endoplasmic reticulum / signal transduction / extracellular exosome / nucleoplasm / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Chen, X. / Ji, X. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 1998 Journal: J.Mol.Biol. / Year: 1998Title: Crystal structure of apo-cellular retinoic acid-binding protein type II (R111M) suggests a mechanism of ligand entry. Authors: Chen, X. / Tordova, M. / Gilliland, G.L. / Wang, L. / Li, Y. / Yan, H. / Ji, X. #1:  Journal: J.Mol.Biol. / Year: 1995 Journal: J.Mol.Biol. / Year: 1995Title: Crystal Structure of Cellular Retinoic Acid Binding Protein I Shows Increased Access to the Binding Cavity due to Formation of an Intermolecular Beta-Sheet Authors: Thompson, J.R. / Bratt, J.M. / Banaszak, L.J. #2:  Journal: Structure / Year: 1994 Journal: Structure / Year: 1994Title: Crystal Structures of Cellular Retinoic Acid Binding Proteins I and II in Complex with All-Trans-Retinoic Acid and a Synthetic Retinoid Authors: Kleywegt, G.J. / Bergfors, T. / Senn, H. / Le Motte, P. / Gsell, B. / Shudo, K. / Jones, T.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1xca.cif.gz 1xca.cif.gz | 72.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1xca.ent.gz pdb1xca.ent.gz | 54.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1xca.json.gz 1xca.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xc/1xca https://data.pdbj.org/pub/pdb/validation_reports/xc/1xca ftp://data.pdbj.org/pub/pdb/validation_reports/xc/1xca ftp://data.pdbj.org/pub/pdb/validation_reports/xc/1xca | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1cbsS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 15555.804 Da / Num. of mol.: 2 / Mutation: R111M Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Cell line: BL21 / Cellular location: CYTOPLASM / Plasmid: BL21 / Cellular location (production host): CYTOPLASM / Production host: Homo sapiens (human) / Cell line: BL21 / Cellular location: CYTOPLASM / Plasmid: BL21 / Cellular location (production host): CYTOPLASM / Production host:  #2: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.35 Å3/Da / Density % sol: 48.6 % | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 8 Details: PROTEIN CONC, 30MG/ML BUFFER 0.1M TRIS, PH 8.0 SALT 0.2M SODIUM ACETATE PRECIPITANT 30% PEG 8000 | ||||||||||||||||||||||||||||||||||||||||
| Crystal | *PLUS | ||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 298 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-D / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU FR-D / Wavelength: 1.5418 |
| Detector | Type: SIEMENS / Detector: AREA DETECTOR / Date: Apr 15, 1995 / Details: COLLIMATOR |
| Radiation | Monochromator: GRAPHITE(002) / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.2→20 Å / Num. obs: 13213 / % possible obs: 81.9 % / Observed criterion σ(I): 0 / Redundancy: 2.75 % / Biso Wilson estimate: 57.5 Å2 / Rmerge(I) obs: 0.08 / Rsym value: 0.059 / Net I/σ(I): 10.4 |
| Reflection shell | Resolution: 2.2→2.28 Å / Redundancy: 1.64 % / Mean I/σ(I) obs: 1.5 / Rsym value: 0.279 / % possible all: 55.9 |
| Reflection | *PLUS Num. measured all: 32588 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1CBS WITH LIGAND AND SOLVENT MOLECULES REMOVED Resolution: 2.3→8 Å / Rfactor Rfree error: 0.0086 / Data cutoff high absF: 100000 / Data cutoff low absF: 0.1 / Isotropic thermal model: UNRESTRAINED / Cross valid method: THROUGHOUT UNTIL LAST FEW CYCLES / σ(F): 2 Details: RESIDUES 24 - 37 OF CHAIN A WERE NOT OBSERVED AND NOT INCLUDED IN THE REFINEMENT; RESTRAINTS BETWEEN THE TWO COPIES OF MOLECULES WERE APPLIED DURING THE INITIAL STAGE OF THE REFINEMENT THE ...Details: RESIDUES 24 - 37 OF CHAIN A WERE NOT OBSERVED AND NOT INCLUDED IN THE REFINEMENT; RESTRAINTS BETWEEN THE TWO COPIES OF MOLECULES WERE APPLIED DURING THE INITIAL STAGE OF THE REFINEMENT THE DISORDERED REGION (RESIDUES 24 - 37 OF CHAIN A) WAS MODELED STEREOCHEMICALLY.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 37.6 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.3→2.4 Å / Rfactor Rfree error: 0.039 / Total num. of bins used: 8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.1 / Classification: refinement X-PLOR / Version: 3.1 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.18 / Rfactor Rwork: 0.18 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj



