[English] 日本語
 Yorodumi
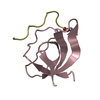
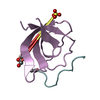
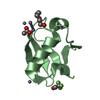
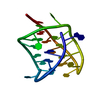
Yorodumi- PDB-1m94: Solution Structure of the Yeast Ubiquitin-Like Modifier Protein Hub1 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1m94 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Solution Structure of the Yeast Ubiquitin-Like Modifier Protein Hub1 | ||||||
 Components Components | Protein YNR032c-a | ||||||
 Keywords Keywords | STRUCTURAL GENOMICS / SIGNALING PROTEIN / ubiquitin-like fold or beta-grasp fold / PSI / Protein Structure Initiative / Northeast Structural Genomics Consortium / NESG | ||||||
| Function / homology |  Function and homology information Function and homology informationcell morphogenesis involved in conjugation with cellular fusion / post-translational protein modification / positive regulation of RNA splicing / mRNA splicing, via spliceosome / protein modification process / protein tag activity / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | SOLUTION NMR / distance geometry, simulated annealing | ||||||
 Authors Authors | Ramelot, T.A. / Cort, J.R. / Yee, A.A. / Semesi, A. / Edwards, A.M. / Arrowsmith, C.H. / Kennedy, M.A. / Northeast Structural Genomics Consortium (NESG) | ||||||
 Citation Citation |  Journal: J.STRUCT.FUNCT.GENOM. / Year: 2003 Journal: J.STRUCT.FUNCT.GENOM. / Year: 2003Title: Solution Structure of the Yeast Ubiquitin-Like Modifier Protein Hub1 Authors: Ramelot, T.A. / Cort, J.R. / Yee, A.A. / Semesi, A. / Edwards, A.M. / Arrowsmith, C.H. / Kennedy, M.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1m94.cif.gz 1m94.cif.gz | 505.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1m94.ent.gz pdb1m94.ent.gz | 426.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1m94.json.gz 1m94.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1m94_validation.pdf.gz 1m94_validation.pdf.gz | 346.2 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1m94_full_validation.pdf.gz 1m94_full_validation.pdf.gz | 467.7 KB | Display | |
| Data in XML |  1m94_validation.xml.gz 1m94_validation.xml.gz | 22.6 KB | Display | |
| Data in CIF |  1m94_validation.cif.gz 1m94_validation.cif.gz | 37.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/m9/1m94 https://data.pdbj.org/pub/pdb/validation_reports/m9/1m94 ftp://data.pdbj.org/pub/pdb/validation_reports/m9/1m94 ftp://data.pdbj.org/pub/pdb/validation_reports/m9/1m94 | HTTPS FTP |
-Related structure data
| Similar structure data | |
|---|---|
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 10454.938 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Gene: YNR032c-a/HUB1 / Plasmid: pET15b / Production host:  |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||||||||||
| NMR details | Text: 1H-13C HSQC on 10% C13, U-15N sample for stereospecific assignments of Leu and Val |
- Sample preparation
Sample preparation
| Details |
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample conditions | Ionic strength: 10 mM NaOAc, 300 mM NaCl / pH: 5 / Pressure: ambient / Temperature: 298 K | |||||||||||||||
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radiation wavelength | Relative weight: 1 | ||||||||||||||||||||
| NMR spectrometer |
|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: distance geometry, simulated annealing / Software ordinal: 1 Details: THE STRUCTURES ARE BASED ON A TOTAL OF 869 RESTRAINTS. SUMMARY OF EXPERIMENTAL CONSTRAINTS: DISTANCE CONSTRAINTS: TOTAL = 783; INTRA-RESIDUE [I=J] = 4; SEQUENTIAL [(I-J)=1] = 229; MEDIUM ...Details: THE STRUCTURES ARE BASED ON A TOTAL OF 869 RESTRAINTS. SUMMARY OF EXPERIMENTAL CONSTRAINTS: DISTANCE CONSTRAINTS: TOTAL = 783; INTRA-RESIDUE [I=J] = 4; SEQUENTIAL [(I-J)=1] = 229; MEDIUM RANGE [1<(I-J)<5] = 159; LONG RANGE [(I-J)>=5] = 333; HYDROGEN BOND CONSTRAINTS = 58 (2 PER H-BOND); NUMBER OF DISTANCE CONSTRAINTS PER RESIDUE = 10.7; DIHEDRAL-ANGLE CONSTRAINTS = 86 (50 PHI, 36 PSI); TOTAL NUMBER OF CONSTRAINTS PER RESIDUE = 11.9; NUMBER OF LONG RANGE CONSTRAINTS PER RESIDUE = 4.6; NUMBER OF STRUCTURES COMPUTED = 20; NUMBER OF STRUCTURES USED = 20. AVERAGE DISTANCE VIOLATIONS >0 ANG = 22.9; AVERAGE R.M.S. DISTANCE VIOLATION = 0.006 ANG; MAXIMUM NUMBER OF DISTANCE VIOLATIONS 28. AVERAGE DIHEDRAL ANGLE VIOLATIONS: >0 DEG = 4.6; MAX NUMBER OF ANGLE VIOLATION = 7 DEG; AVERAGE R.M.S. ANGLE VIOLATION = 0.14 DEG. RMSD VALUES: BACKBONE ATOMS (N,C,C',O) = 0.61 ANG; ALL HEAVY ATOMS = 1.17 ANG; PROCHECK: MOST FAVORED REGIONS = 92%; ADDITIONAL ALLOWED REGIONS = 4%; GENEROUSLY ALLOWED REGIONS = 2%; DISALLOWED REGIONS = 2%. | ||||||||||||||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: all calculated structures submitted Conformers calculated total number: 20 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller








 PDBj
PDBj X-PLOR
X-PLOR