[English] 日本語
 Yorodumi
Yorodumi- PDB-1gau: SOLUTION STRUCTURE OF THE SPECIFIC DNA COMPLEX OF THE ZINC CONTAI... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1gau | ||||||
|---|---|---|---|---|---|---|---|
| Title | SOLUTION STRUCTURE OF THE SPECIFIC DNA COMPLEX OF THE ZINC CONTAINING DNA BINDING DOMAIN OF THE ERYTHROID TRANSCRIPTION FACTOR GATA-1 BY MULTIDIMENSIONAL NMR | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSCRIPTION/DNA / TRANSCRIPTION-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology informationcell fate commitment / transcription coactivator binding / DNA-binding transcription factor activity, RNA polymerase II-specific / RNA polymerase II cis-regulatory region sequence-specific DNA binding / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / zinc ion binding / nucleus Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | SOLUTION NMR | ||||||
 Authors Authors | Clore, G.M. / Omichinski, J.G. / Gronenborn, A.M. | ||||||
 Citation Citation |  Journal: Science / Year: 1993 Journal: Science / Year: 1993Title: NMR structure of a specific DNA complex of Zn-containing DNA binding domain of GATA-1. Authors: Omichinski, J.G. / Clore, G.M. / Schaad, O. / Felsenfeld, G. / Trainor, C. / Appella, E. / Stahl, S.J. / Gronenborn, A.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1gau.cif.gz 1gau.cif.gz | 869 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1gau.ent.gz pdb1gau.ent.gz | 715.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1gau.json.gz 1gau.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1gau_validation.pdf.gz 1gau_validation.pdf.gz | 370.3 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1gau_full_validation.pdf.gz 1gau_full_validation.pdf.gz | 874 KB | Display | |
| Data in XML |  1gau_validation.xml.gz 1gau_validation.xml.gz | 53.2 KB | Display | |
| Data in CIF |  1gau_validation.cif.gz 1gau_validation.cif.gz | 86.1 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ga/1gau https://data.pdbj.org/pub/pdb/validation_reports/ga/1gau ftp://data.pdbj.org/pub/pdb/validation_reports/ga/1gau ftp://data.pdbj.org/pub/pdb/validation_reports/ga/1gau | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 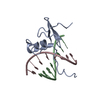
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: DNA chain | Mass: 2443.657 Da / Num. of mol.: 1 / Source method: obtained synthetically |
|---|---|
| #2: DNA chain | Mass: 2407.601 Da / Num. of mol.: 1 / Source method: obtained synthetically |
| #3: Protein | Mass: 6798.865 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR |
|---|
- Sample preparation
Sample preparation
| Crystal grow | *PLUS Method: other / Details: NMR |
|---|
- Processing
Processing
| Refinement | Software ordinal: 1 Details: DETAILS OF THE STRUCTURE DETERMINATION AND ALL STRUCTURAL STATISTICS ARE GIVEN IN THE REFERENCE CITED ON THE *JRNL* RECORDS ABOVE (I.E. AGREEMENT WITH EXPERIMENTAL RESTRAINTS, DEVIATIONS ...Details: DETAILS OF THE STRUCTURE DETERMINATION AND ALL STRUCTURAL STATISTICS ARE GIVEN IN THE REFERENCE CITED ON THE *JRNL* RECORDS ABOVE (I.E. AGREEMENT WITH EXPERIMENTAL RESTRAINTS, DEVIATIONS FROM IDEALITY FOR BOND LENGTHS, ANGLES, PLANES AND CHIRALITY, NON-BONDED CONTACTS, ATOMIC RMS DIFFERENCES BETWEEN THE CALCULATED STRUCTURES). THE STRUCTURES ARE BASED ON A TOTAL OF 1740 EXPERIMENTAL NMR RESTRAINTS COMPRISING: 1444 INTERPROTON DISTANCE RESTRAINTS DERIVED FROM NOE MEASUREMENTS; AND 296 TORSION ANGLE RESTRAINTS. THE NOE RESTRAINTS ARE SUBDIVIDED AS FOLLOWS: (A) WITHIN THE PROTEIN: 242 INTERRESIDUE SEQUENTIAL (|I-J|=1); 161 INTERRESIDUE SHORT RANGE (1(LESS THAN)|I-J|(LESS THAN)=5); 182 INTERRESIDUE LONG RANGE (|I-J|(GREATER THAN)5); AND 334 INTRARESIDUE. (B) WITHIN THE DNA: 157 INTRARESIDUE; 180 SEQUENTIAL INTRASTRAND; 34 INTERSTRAND; AND 37 H-BONDS (C) BETWEEN PROTEIN AND DNA: 117. THE TORSION ANGLE RESTRAINTS ARE SUBDIVIDED AS FOLLOWS: 144 ANGLES FOR THE PROTEIN (58 PHI, 56 PSI, 26 CHI1 AND 4 CHI2) AND 152 FOR THE DNA. THE TORSION ANGLE RESTRAINTS FOR THE DNA COMPRISE LOOSE RESTRAINTS ON THE BACKBONE TORSION ANGLES ALPHA, BETA, GAMMA, EPSILON AND ZETA TO PREVENT PROBLEMS ASSOCIATED WITH LOCAL MIRROR IMAGES. THE METHOD USED TO DETERMINE THE STRUCTURES IS THE HYBRID METRIC MATRIX DISTANCE GEOMETRY-DYNAMICAL SIMULATED ANNEALING METHOD [M.NILGES, G.M.CLORE, AND A.M.GRONENBORN FEBS LETT. 229, 317-324 (1988)]. A TOTAL OF 30 STRUCTURES WERE CALCULATED. THE ATOMIC RMS DISTRIBUTION ABOUT THE MEAN COORDINATE POSITIONS FOR RESIDUES 2 - 59 OF THE PROTEIN AND BASE PAIRS 6 - 13 OF THE DNA IS 0.70 (+/-0.13) A FOR THE BACKBONE ATOMS OF THE PROTEIN AND ALL ATOMS OF THE DNA, AND 1.13 (+/-0.08) A FOR ALL ATOMS OF THE PROTEIN AND DNA. THE N-TERMINUS (RESIDUE 1) AND C-TERMINUS (RESIDUES 60 - 66) ARE DISORDERED. THE ORIENTATION OF THE FIRST 5 AND LAST 3 BASE PAIRS OF THE 16MER DNA DUPLEX, WHICH ARE NOT IN CONTACT WITH THE DNA, IS POORLY DEFINED WITH RESPECT TO THE CORE OF THE COMPLEX. CONSEQUENTLY, ONLY THE COORDINATES OF THE COMPLEX PROPER HAVE BEEN DEPOSITED: I.E. RESIDUES 1 - 60 OF THE PROTEIN AND BASE PAIRS 6 - 13 OF THE DNA. THE COORDINATES OF THE 30 INDIVIDUAL SA STRUCTURES ARE PRESENTED AS MODELS 1 TO 30. THE QUANTITY IN COLUMNS 61 - OF THESE MODELS HAS NO MEANING. THE COORDINATES OF THE RESTRAINED MINIMIZED STRUCTURE ARE PRESENTED IN PROTEIN DATA BANK ENTRY 1GAT. THE (SA)R RESTRAINED MINIMIZED MEAN STRUCTURE WAS DERIVED BY AVERAGING THE COORDINATES OF THE INDIVIDUAL SA STRUCTURES (BEST FITTED TO RESIDUES 2 - 59 OF THE PROTEIN AND BASE PAIRS 6 - 13 OF THE DNA), AND SUBJECTING THE RESULTING COORDINATES TO RESTRAINED MINIMIZATION. THE QUANTITY PRESENTED IN COLUMNS 61 - 66 OF MEAN STRUCTURE, PRESENTED IN PROTEIN DATA BANK ENTRY 1GAT, REPRESENTS THE ATOMIC RMS DEVIATIONS OF THE 30 INDIVIDUAL SA STRUCTURES ABOUT THE MEAN STRUCTURE. |
|---|---|
| NMR ensemble | Conformers submitted total number: 30 |
 Movie
Movie Controller
Controller













 PDBj
PDBj
