[English] 日本語
 Yorodumi
Yorodumi- PDB-1fjm: Protein serine/threonine phosphatase-1 (alpha isoform, type 1) co... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1fjm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
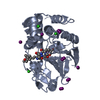
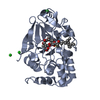
| Title | Protein serine/threonine phosphatase-1 (alpha isoform, type 1) complexed with microcystin-LR toxin | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / HYDROLASE / TOXIN / HYDROLASE-HYDROLASE INHIBITOR complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of translational initiation by eIF2 alpha dephosphorylation / protein serine/threonine phosphatase activity => GO:0004722 / protein serine/threonine phosphatase activity => GO:0004722 / fatty acid derivative binding / beta-catenin destruction complex disassembly / cell cycle / regulation of glycogen catabolic process / peptidyl-serine dephosphorylation / regulation of glycogen biosynthetic process / PTW/PP1 phosphatase complex ...regulation of translational initiation by eIF2 alpha dephosphorylation / protein serine/threonine phosphatase activity => GO:0004722 / protein serine/threonine phosphatase activity => GO:0004722 / fatty acid derivative binding / beta-catenin destruction complex disassembly / cell cycle / regulation of glycogen catabolic process / peptidyl-serine dephosphorylation / regulation of glycogen biosynthetic process / PTW/PP1 phosphatase complex / protein phosphatase type 1 complex / peptidyl-threonine dephosphorylation / glycogen granule / RNA polymerase II promoter clearance / RNA polymerase II CTD heptapeptide repeat S5 phosphatase activity / cadherin binding involved in cell-cell adhesion / protein phosphatase 1 binding / regulation of translational initiation in response to stress / positive regulation of extrinsic apoptotic signaling pathway in absence of ligand / dephosphorylation / regulation of canonical Wnt signaling pathway / regulation of translational initiation / glycogen metabolic process / protein dephosphorylation / protein-serine/threonine phosphatase / entrainment of circadian clock by photoperiod / branching morphogenesis of an epithelial tube / Triglyceride catabolism / protein serine/threonine phosphatase activity / phosphatase activity / negative regulation of transcription elongation by RNA polymerase II / viral process / transition metal ion binding / protein phosphatase activator activity / DARPP-32 events / negative regulation of protein binding / ribonucleoprotein complex binding / : / phosphoprotein phosphatase activity / lung development / Downregulation of TGF-beta receptor signaling / adherens junction / circadian regulation of gene expression / positive regulation of transcription elongation by RNA polymerase II / regulation of circadian rhythm / response to lead ion / positive regulation of canonical Wnt signaling pathway / presynapse / dendritic spine / perikaryon / iron ion binding / cell division / nucleolus / glutamatergic synapse / extracellular exosome / nucleoplasm / metal ion binding / nucleus / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |   Microcystis aeruginosa (bacteria) Microcystis aeruginosa (bacteria) | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.1 Å X-RAY DIFFRACTION / Resolution: 2.1 Å | |||||||||
 Authors Authors | Goldberg, J. / Nairn, A.C. / Kuriyan, J. | |||||||||
 Citation Citation |  Journal: Nature / Year: 1995 Journal: Nature / Year: 1995Title: Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Authors: Goldberg, J. / Huang, H.B. / Kwon, Y.G. / Greengard, P. / Nairn, A.C. / Kuriyan, J. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1fjm.cif.gz 1fjm.cif.gz | 137.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1fjm.ent.gz pdb1fjm.ent.gz | 106.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1fjm.json.gz 1fjm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fj/1fjm https://data.pdbj.org/pub/pdb/validation_reports/fj/1fjm ftp://data.pdbj.org/pub/pdb/validation_reports/fj/1fjm ftp://data.pdbj.org/pub/pdb/validation_reports/fj/1fjm | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (0.251034, -0.966528, 0.052974), Vector: |
- Components
Components
| #1: Protein | Mass: 37558.051 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  References: UniProt: P08129, UniProt: P62139*PLUS, protein-serine/threonine phosphatase #2: Protein/peptide | #3: Chemical | ChemComp-MN / #4: Chemical | #5: Water | ChemComp-HOH / | Compound details | ELECTRON DENSITY MAPS SHOW THAT RESIDUE CYSTEINE 127 IS OXIDIZED, WITH A TERMINAL SULPHINYL OR ...ELECTRON DENSITY MAPS SHOW THAT RESIDUE CYSTEINE 127 IS OXIDIZED, WITH A TERMINAL SULPHINYL OR SULPHONYL GROUP. HOWEVER, THE TERMINAL OXYGEN ATOMS OF THE SIDE CHAIN HAVE NOT BEEN MODELLED. | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.12 Å3/Da / Density % sol: 41.86 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 7 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 2.1 Å / Lowest resolution: 20 Å / Num. obs: 38768 / % possible obs: 99.5 % / Num. measured all: 180676 / Rmerge(I) obs: 0.056 |
| Reflection shell | *PLUS % possible obs: 97.2 % / Rmerge(I) obs: 0.136 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.1→6 Å / Rfactor Rwork: 0.178 / Rfactor obs: 0.178 / σ(F): 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→6 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Num. reflection all: 36974 / Rfactor all: 0.178 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller










 PDBj
PDBj