[English] 日本語
 Yorodumi
Yorodumi- PDB-1ent: X-RAY ANALYSES OF ASPARTIC PROTEINASES. THE THREE-DIMENSIONAL STR... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ent | ||||||
|---|---|---|---|---|---|---|---|
| Title | X-RAY ANALYSES OF ASPARTIC PROTEINASES. THE THREE-DIMENSIONAL STRUCTURE AT 2.1 ANGSTROMS RESOLUTION OF ENDOTHIAPEPSIN | ||||||
 Components Components | ENDOTHIAPEPSIN | ||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / ACID PROTEINASE / HYDROLASE-HYDROLASE INHIBITOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  Cryphonectria parasitica (chestnut blight fungus) Cryphonectria parasitica (chestnut blight fungus) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.9 Å X-RAY DIFFRACTION / Resolution: 1.9 Å | ||||||
 Authors Authors | Blundell, T.L. / Dealwis, C.G. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 1990 Journal: J.Mol.Biol. / Year: 1990Title: X-ray analyses of aspartic proteinases. The three-dimensional structure at 2.1 A resolution of endothiapepsin. Authors: Blundell, T.L. / Jenkins, J.A. / Sewell, B.T. / Pearl, L.H. / Cooper, J.B. / Tickle, I.J. / Veerapandian, B. / Wood, S.P. #1:  Journal: J.Mol.Biol. / Year: 1990 Journal: J.Mol.Biol. / Year: 1990Title: X-Ray Analyses of Aspartic Proteinases. III Three-Dimensional Structure of Endothiapepsin Complexed with a Transition-State Isostere Inhibitor of Renin at 1.6 Angstroms Resolution Authors: Veerapandian, B. / Cooper, J.B. / Sali, A. / Blundell, T.L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ent.cif.gz 1ent.cif.gz | 77.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ent.ent.gz pdb1ent.ent.gz | 55.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ent.json.gz 1ent.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1ent_validation.pdf.gz 1ent_validation.pdf.gz | 462.2 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1ent_full_validation.pdf.gz 1ent_full_validation.pdf.gz | 468.5 KB | Display | |
| Data in XML |  1ent_validation.xml.gz 1ent_validation.xml.gz | 8.9 KB | Display | |
| Data in CIF |  1ent_validation.cif.gz 1ent_validation.cif.gz | 14.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/en/1ent https://data.pdbj.org/pub/pdb/validation_reports/en/1ent ftp://data.pdbj.org/pub/pdb/validation_reports/en/1ent ftp://data.pdbj.org/pub/pdb/validation_reports/en/1ent | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: CIS PROLINE - PRO E 23 / 2: CIS PROLINE - PRO E 133 |
- Components
Components
| #1: Protein | Mass: 33813.855 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Cryphonectria parasitica (chestnut blight fungus) Cryphonectria parasitica (chestnut blight fungus)References: UniProt: P11838, endothiapepsin | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #2: Chemical | ChemComp-0EM / 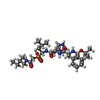  Type: peptide-like, Peptide-like / Class: Inhibitor / Mass: 663.765 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C32H52N6O7P Type: peptide-like, Peptide-like / Class: Inhibitor / Mass: 663.765 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C32H52N6O7PReferences: N-(tert-butoxycarbonyl)-L-phenylalanyl-N-{(1S)-1-[(R)-hydroxy(2-{[(2S)-2-methylbutyl]amino}-2-oxoethyl)phosphoryl]-3- methylbutyl}-3-(1H-imidazol-3-ium-4-yl)-L-alaninamide | ||||||||
| #3: Chemical | | #4: Water | ChemComp-HOH / | Compound details | THE PHOSPHINATE INHIBITOR PD130328 COMPLEXED WITH ENDOTHIAPEPSIN HAS TWO SHORT HYDROGEN BONDS OF 2. ...THE PHOSPHINAT | Has protein modification | Y | Sequence details | THE COMPLETE SEQUENCE WAS DETERMINED BY V. PEDERSEN AS TRYPTIC FRAGMENTS WHICH WERE ALIGNED IN THE ...THE COMPLETE SEQUENCE WAS DETERMINED | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.01 Å3/Da / Density % sol: 38.8 % | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 4.6 / Method: unknown | ||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 2.1 Å / Num. obs: 19356 / Num. measured all: 44358 / Rmerge(I) obs: 0.074 |
- Processing
Processing
| Software | Name: RESTRAIN / Classification: refinement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Rfactor obs: 0.17 / Highest resolution: 1.9 Å Details: THE QUANTITY GIVEN IN THE TEMPERATURE FACTOR FIELD OF THE *ATOM* AND *HETATM* RECORDS BELOW IS U**2, WHICH IS THE MEAN-SQUARE AMPLITUDE OF ATOMIC VIBRATION. THE TEMPERATURE FACTOR, B, CAN BE ...Details: THE QUANTITY GIVEN IN THE TEMPERATURE FACTOR FIELD OF THE *ATOM* AND *HETATM* RECORDS BELOW IS U**2, WHICH IS THE MEAN-SQUARE AMPLITUDE OF ATOMIC VIBRATION. THE TEMPERATURE FACTOR, B, CAN BE DERIVED BY THE FOLLOWING RELATION - B = 8 * (PI)**2 * U**2. IT IS AN INDICATION OF POSSIBLE ERRORS IN THE REFINEMENT THAT SOME ARE SLIGHTLY NEGATIVE. | ||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 1.9 Å
| ||||||||||||
| Refine LS restraints |
| ||||||||||||
| Software | *PLUS Name: RESTRAIN / Classification: refinement | ||||||||||||
| Refinement | *PLUS Highest resolution: 2.1 Å / Rfactor obs: 0.178 / Lowest resolution: 20 Å / Rfactor Rwork: 0.178 | ||||||||||||
| Solvent computation | *PLUS | ||||||||||||
| Displacement parameters | *PLUS Biso mean: 18.9 Å2 |
 Movie
Movie Controller
Controller






















 PDBj
PDBj



