+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9864 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Asymmetry reconstruction of HSV-1 virion | |||||||||
 Map data Map data | HSV-1 virion asymmetry reconstruction | |||||||||
 Sample Sample |
| |||||||||
| Biological species |   Human herpesvirus 1 strain KOS Human herpesvirus 1 strain KOS | |||||||||
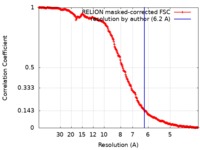
| Method | single particle reconstruction / cryo EM / Resolution: 6.2 Å | |||||||||
 Authors Authors | Liu YT / Jih J / Dai X / Bi GQ / Zhou ZH | |||||||||
| Funding support |  China, China,  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Cryo-EM structures of herpes simplex virus type 1 portal vertex and packaged genome. Authors: Yun-Tao Liu / Jonathan Jih / Xinghong Dai / Guo-Qiang Bi / Z Hong Zhou /   Abstract: Herpesviruses are enveloped viruses that are prevalent in the human population and are responsible for diverse pathologies, including cold sores, birth defects and cancers. They are characterized by ...Herpesviruses are enveloped viruses that are prevalent in the human population and are responsible for diverse pathologies, including cold sores, birth defects and cancers. They are characterized by a highly pressurized pseudo-icosahedral capsid-with triangulation number (T) equal to 16-encapsidating a tightly packed double-stranded DNA (dsDNA) genome. A key process in the herpesvirus life cycle involves the recruitment of an ATP-driven terminase to a unique portal vertex to recognize, package and cleave concatemeric dsDNA, ultimately giving rise to a pressurized, genome-containing virion. Although this process has been studied in dsDNA phages-with which herpesviruses bear some similarities-a lack of high-resolution in situ structures of genome-packaging machinery has prevented the elucidation of how these multi-step reactions, which require close coordination among multiple actors, occur in an integrated environment. To better define the structural basis of genome packaging and organization in herpes simplex virus type 1 (HSV-1), we developed sequential localized classification and symmetry relaxation methods to process cryo-electron microscopy (cryo-EM) images of HSV-1 virions, which enabled us to decouple and reconstruct hetero-symmetric and asymmetric elements within the pseudo-icosahedral capsid. Here we present in situ structures of the unique portal vertex, genomic termini and ordered dsDNA coils in the capsid spooled around a disordered dsDNA core. We identify tentacle-like helices and a globular complex capping the portal vertex that is not observed in phages, indicative of herpesvirus-specific adaptations in the DNA-packaging process. Finally, our atomic models of portal vertex elements reveal how the fivefold-related capsid accommodates symmetry mismatch imparted by the dodecameric portal-a longstanding mystery in icosahedral viruses-and inform possible DNA-sequence recognition and headful-sensing pathways involved in genome packaging. This work showcases how to resolve symmetry-mismatched elements in a large eukaryotic virus and provides insights into the mechanisms of herpesvirus genome packaging. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9864.map.gz emd_9864.map.gz | 1.3 GB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9864-v30.xml emd-9864-v30.xml emd-9864.xml emd-9864.xml | 9.7 KB 9.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_9864_fsc.xml emd_9864_fsc.xml | 25.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_9864.png emd_9864.png | 216.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9864 http://ftp.pdbj.org/pub/emdb/structures/EMD-9864 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9864 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9864 | HTTPS FTP |
-Related structure data
| Related structure data |  9860C  9861C  9862C  9863C  6od7C  6odmC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_9864.map.gz / Format: CCP4 / Size: 1.4 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9864.map.gz / Format: CCP4 / Size: 1.4 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HSV-1 virion asymmetry reconstruction | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human herpesvirus 1 strain KOS
| Entire | Name:   Human herpesvirus 1 strain KOS Human herpesvirus 1 strain KOS |
|---|---|
| Components |
|
-Supramolecule #1: Human herpesvirus 1 strain KOS
| Supramolecule | Name: Human herpesvirus 1 strain KOS / type: virus / ID: 1 / Parent: 0 / NCBI-ID: 10306 / Sci species name: Human herpesvirus 1 strain KOS / Sci species strain: KOS / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE Details: The sample was manually blotted and frozen with a homemade plunger... |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 14000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)