+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | TPeA-bound closed MthK channel in nanodisc | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ion channel / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmonoatomic cation transmembrane transporter activity / potassium ion transport / metal ion binding / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Methanothermobacter thermautotrophicus (archaea) Methanothermobacter thermautotrophicus (archaea) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Fan C / Nimigean CM | |||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2024 Journal: Nat Chem Biol / Year: 2024Title: Calcium-gated potassium channel blockade via membrane-facing fenestrations. Authors: Chen Fan / Emelie Flood / Nattakan Sukomon / Shubhangi Agarwal / Toby W Allen / Crina M Nimigean /    Abstract: Quaternary ammonium blockers were previously shown to bind in the pore to block both open and closed conformations of large-conductance calcium-activated potassium (BK and MthK) channels. Because ...Quaternary ammonium blockers were previously shown to bind in the pore to block both open and closed conformations of large-conductance calcium-activated potassium (BK and MthK) channels. Because blocker entry was assumed through the intracellular entryway (bundle crossing), closed-pore access suggested that the gate was not at the bundle crossing. Structures of closed MthK, a Methanobacterium thermoautotrophicum homolog of BK channels, revealed a tightly constricted intracellular gate, leading us to investigate the membrane-facing fenestrations as alternative pathways for blocker access directly from the membrane. Atomistic free energy simulations showed that intracellular blockers indeed access the pore through the fenestrations, and a mutant channel with narrower fenestrations displayed no closed-state TPeA block at concentrations that blocked the wild-type channel. Apo BK channels display similar fenestrations, suggesting that blockers may use them as access paths into closed channels. Thus, membrane fenestrations represent a non-canonical pathway for selective targeting of specific channel conformations, opening novel ways to selectively drug BK channels. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9406.map.gz emd_9406.map.gz | 4.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9406-v30.xml emd-9406-v30.xml emd-9406.xml emd-9406.xml | 12.6 KB 12.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_9406.png emd_9406.png | 193.9 KB | ||
| Filedesc metadata |  emd-9406.cif.gz emd-9406.cif.gz | 5.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9406 http://ftp.pdbj.org/pub/emdb/structures/EMD-9406 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9406 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9406 | HTTPS FTP |
-Related structure data
| Related structure data |  5bkjMC  9405C  9407C  5bkiC  5bkkC  8djbC  8fz7C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9406.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9406.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : MthK
| Entire | Name: MthK |
|---|---|
| Components |
|
-Supramolecule #1: MthK
| Supramolecule | Name: MthK / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Methanothermobacter thermautotrophicus (archaea) Methanothermobacter thermautotrophicus (archaea) |
-Macromolecule #1: Calcium-gated potassium channel MthK
| Macromolecule | Name: Calcium-gated potassium channel MthK / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Methanothermobacter thermautotrophicus (archaea) Methanothermobacter thermautotrophicus (archaea) |
| Molecular weight | Theoretical: 37.35616 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MVLVIEIIRK HLPRVLKVPA TRILLLVLAV IIYGTAGFHF IEGESWTVSL YWTFVTIATV GYGDYSPSTP LGMYFTVTLI VLGIGTFAV AVERLLEFLI NREQMKLMGL IDVAKSRHVV ICGWSESTLE CLRELRGSEV FVLAEDENVR KKVLRSGANF V HGDPTRVS ...String: MVLVIEIIRK HLPRVLKVPA TRILLLVLAV IIYGTAGFHF IEGESWTVSL YWTFVTIATV GYGDYSPSTP LGMYFTVTLI VLGIGTFAV AVERLLEFLI NREQMKLMGL IDVAKSRHVV ICGWSESTLE CLRELRGSEV FVLAEDENVR KKVLRSGANF V HGDPTRVS DLEKANVRGA RAVIVDLESD SETIHCILGI RKIDESVRII AEAERYENIE QLRMAGADQV ISPFVISGRL MS RSIDDGY EAMFVQDVLA EESTRRMVEV PIPEGSKLEG VSVLDADIHD VTGVIIIGVG RGDELIIDPP RDYSFRAGDI ILG IGKPEE IERLKNYISA UniProtKB: Calcium-gated potassium channel MthK |
-Macromolecule #2: (1R)-2-{[(S)-{[(2S)-2,3-dihydroxypropyl]oxy}(hydroxy)phosphoryl]o...
| Macromolecule | Name: (1R)-2-{[(S)-{[(2S)-2,3-dihydroxypropyl]oxy}(hydroxy)phosphoryl]oxy}-1-[(hexadecanoyloxy)methyl]ethyl (9Z)-octadec-9-enoate type: ligand / ID: 2 / Number of copies: 4 / Formula: PGW |
|---|---|
| Molecular weight | Theoretical: 749.007 Da |
-Macromolecule #3: POTASSIUM ION
| Macromolecule | Name: POTASSIUM ION / type: ligand / ID: 3 / Number of copies: 2 / Formula: K |
|---|---|
| Molecular weight | Theoretical: 39.098 Da |
-Macromolecule #4: 1-(tripentyl-$l^{4}-azanyl)pentane
| Macromolecule | Name: 1-(tripentyl-$l^{4}-azanyl)pentane / type: ligand / ID: 4 / Number of copies: 1 / Formula: YQ1 |
|---|---|
| Molecular weight | Theoretical: 298.57 Da |
| Chemical component information | 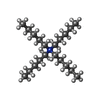 ChemComp-YQ1: |
-Macromolecule #5: water
| Macromolecule | Name: water / type: ligand / ID: 5 / Number of copies: 4 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 52.23 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















