[English] 日本語
 Yorodumi
Yorodumi- EMDB-8965: Influenza hemagglutinin (HA) trimer reconstruction at 4.1 Angstro... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8965 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Influenza hemagglutinin (HA) trimer reconstruction at 4.1 Angstrom resolution using particles from micrographs tilted at 40 degrees | |||||||||
 Map data Map data | Influenza hemagglutinin (HA) trimer reconstruction at 4.1 Angstrom resolution using particles from micrographs tilted at 40 degrees, primary map | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | |||||||||
 Authors Authors | Su M | |||||||||
 Citation Citation |  Journal: J Struct Biol / Year: 2019 Journal: J Struct Biol / Year: 2019Title: goCTF: Geometrically optimized CTF determination for single-particle cryo-EM. Authors: Min Su /  Abstract: Preferred particle orientation represents a recurring problem in single-particle cryogenic electron microcopy (cryo-EM). A specimen-independent approach through tilting has been attempted to increase ...Preferred particle orientation represents a recurring problem in single-particle cryogenic electron microcopy (cryo-EM). A specimen-independent approach through tilting has been attempted to increase particle orientation coverage, thus minimizing anisotropic three-dimensional (3D) reconstruction. However, focus gradient is a critical issue hindering tilt applications from being a general practice in single-particle cryo-EM. The present study describes a newly developed geometrically optimized approach, goCTF, to reliably determine the global focus gradient. A novel strategy of determining contrast transfer function (CTF) parameters from a sector of the signal preserved power spectrum is applied to increase reliability. Subsequently, per-particle based local focus refinement is conducted in an iterative manner to further improve the defocus accuracy. Novel diagnosis methods using a standard deviation defocus plot and goodness of fit heatmap have also been proposed to evaluate CTF fitting quality prior to 3D refinement. In a benchmark study, goCTF processed a published single-particle cryo-EM dataset for influenza hemagglutinin trimer collected at a 40-degree specimen tilt. The resulting 3D reconstruction map was improved from 4.1 Å to 3.7 Å resolution. The goCTF program is built on the open-source code of CTFFIND4, which adopts a consistent user interface for ease of use. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8965.map.gz emd_8965.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8965-v30.xml emd-8965-v30.xml emd-8965.xml emd-8965.xml | 7.7 KB 7.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8965.png emd_8965.png | 48.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8965 http://ftp.pdbj.org/pub/emdb/structures/EMD-8965 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8965 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8965 | HTTPS FTP |
-Related structure data
| Related structure data |  8966C  8967  8968 C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_8965.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8965.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Influenza hemagglutinin (HA) trimer reconstruction at 4.1 Angstrom resolution using particles from micrographs tilted at 40 degrees, primary map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.31 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
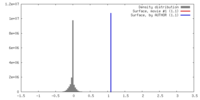
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Influenza hemagglutinin (HA) trimer
| Entire | Name: Influenza hemagglutinin (HA) trimer |
|---|---|
| Components |
|
-Supramolecule #1: Influenza hemagglutinin (HA) trimer
| Supramolecule | Name: Influenza hemagglutinin (HA) trimer / type: organelle_or_cellular_component / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 150 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 82.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 130000 |
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)