+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8698 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | EBOV GPdMuc:ADI-16061 | |||||||||
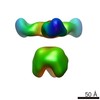
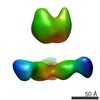
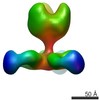
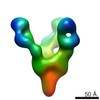
 Map data Map data | Complex of recombinant Ebola virus glycoprotein and recombinant human IgG1 Fab ADI-16061 from survivor of infection. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 25.3 Å | |||||||||
 Authors Authors | Murin CD / Ward AB / Turner HL | |||||||||
 Citation Citation |  Journal: Cell / Year: 2017 Journal: Cell / Year: 2017Title: Antibodies from a Human Survivor Define Sites of Vulnerability for Broad Protection against Ebolaviruses. Authors: Anna Z Wec / Andrew S Herbert / Charles D Murin / Elisabeth K Nyakatura / Dafna M Abelson / J Maximilian Fels / Shihua He / Rebekah M James / Marc-Antoine de La Vega / Wenjun Zhu / Russell R ...Authors: Anna Z Wec / Andrew S Herbert / Charles D Murin / Elisabeth K Nyakatura / Dafna M Abelson / J Maximilian Fels / Shihua He / Rebekah M James / Marc-Antoine de La Vega / Wenjun Zhu / Russell R Bakken / Eileen Goodwin / Hannah L Turner / Rohit K Jangra / Larry Zeitlin / Xiangguo Qiu / Jonathan R Lai / Laura M Walker / Andrew B Ward / John M Dye / Kartik Chandran / Zachary A Bornholdt /   Abstract: Experimental monoclonal antibody (mAb) therapies have shown promise for treatment of lethal Ebola virus (EBOV) infections, but their species-specific recognition of the viral glycoprotein (GP) has ...Experimental monoclonal antibody (mAb) therapies have shown promise for treatment of lethal Ebola virus (EBOV) infections, but their species-specific recognition of the viral glycoprotein (GP) has limited their use against other divergent ebolaviruses associated with human disease. Here, we mined the human immune response to natural EBOV infection and identified mAbs with exceptionally potent pan-ebolavirus neutralizing activity and protective efficacy against three virulent ebolaviruses. These mAbs recognize an inter-protomer epitope in the GP fusion loop, a critical and conserved element of the viral membrane fusion machinery, and neutralize viral entry by targeting a proteolytically primed, fusion-competent GP intermediate (GP) generated in host cell endosomes. Only a few somatic hypermutations are required for broad antiviral activity, and germline-approximating variants display enhanced GP recognition, suggesting that such antibodies could be elicited more efficiently with suitably optimized GP immunogens. Our findings inform the development of both broadly effective immunotherapeutics and vaccines against filoviruses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8698.map.gz emd_8698.map.gz | 758.8 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8698-v30.xml emd-8698-v30.xml emd-8698.xml emd-8698.xml | 14 KB 14 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8698.png emd_8698.png | 30.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8698 http://ftp.pdbj.org/pub/emdb/structures/EMD-8698 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8698 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8698 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_8698.map.gz / Format: CCP4 / Size: 1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8698.map.gz / Format: CCP4 / Size: 1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Complex of recombinant Ebola virus glycoprotein and recombinant human IgG1 Fab ADI-16061 from survivor of infection. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : EBOVGPdMuc:ADI-16061
| Entire | Name: EBOVGPdMuc:ADI-16061 |
|---|---|
| Components |
|
-Supramolecule #1: EBOVGPdMuc:ADI-16061
| Supramolecule | Name: EBOVGPdMuc:ADI-16061 / type: complex / ID: 1 / Parent: 0 Details: Complex of recombinant Ebola virus glycoprotein and recombinant human IgG1 Fab from survivor of infection. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: HEK 293F / Recombinant plasmid: pPPI4 Homo sapiens (human) / Recombinant cell: HEK 293F / Recombinant plasmid: pPPI4 |
-Supramolecule #2: EBOV GPdMuc
| Supramolecule | Name: EBOV GPdMuc / type: complex / ID: 2 / Parent: 1 Details: Recombinant mucin-deleted Ebola virus glycoprotein expressed in mammalian cells, 293F. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: HEK 293F / Recombinant plasmid: pPPI4 Homo sapiens (human) / Recombinant cell: HEK 293F / Recombinant plasmid: pPPI4 |
-Supramolecule #3: ADI-16061 Fab
| Supramolecule | Name: ADI-16061 Fab / type: complex / ID: 3 / Parent: 1 Details: Fab fragment generated by proteolytic cleavage of IgG antibody, expressed in plant system. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.03 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: Solutions were made from a 10X stock and filtered sterilized. | |||||||||
| Staining | Type: NEGATIVE / Material: Uranyl Formate Details: Negatively stained EM specimens were prepared using a side-blotting technique and uranyl-formate stain. | |||||||||
| Grid | Model: Protochip / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Atmosphere: OTHER | |||||||||
| Details | Monodisperse sample deposited on negative stain grid. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI SPIRIT |
|---|---|
| Image recording | Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Number grids imaged: 1 / Number real images: 53 / Average electron dose: 2.0 e/Å2 |
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC |
| Experimental equipment |  Model: Tecnai Spirit / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



 UCSF Chimera
UCSF Chimera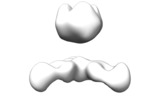




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















