[English] 日本語
 Yorodumi
Yorodumi- EMDB-7969: 3D structure of the native alpha-crystallin from bovine eye lens. -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7969 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 3D structure of the native alpha-crystallin from bovine eye lens. | |||||||||
 Map data Map data | Native alpha-crystallin from bovine eye lens. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 21.0 Å | |||||||||
 Authors Authors | Ryazantsev SN / Poliansky NB / Chebotareva NA / Muranov KO | |||||||||
 Citation Citation |  Journal: Int J Biol Macromol / Year: 2018 Journal: Int J Biol Macromol / Year: 2018Title: 3D structure of the native α-crystallin from bovine eye lens. Authors: Sergey N Ryazantsev / Nikolai B Poliansky / Natalia A Chebotareva / Konstantin O Muranov /   Abstract: α-Crystallin is the major eye lens protein that has been shown to support lens transparency by preventing the aggregation of lens proteins. The 3D structure of α-crystallin is largely unknown. ...α-Crystallin is the major eye lens protein that has been shown to support lens transparency by preventing the aggregation of lens proteins. The 3D structure of α-crystallin is largely unknown. Electron microscopy, single-particle 3D reconstruction, size exclusion chromatography, dynamic light scattering, and analytical ultracentrifugation were used to study the structure of the native α-crystallin. Native α-crystallin has a wide distribution in size. The shape of mass distribution is temperature-dependent, but the oligomers with a sedimentation coefficient of ~22 S (750-830 kDa) strongly prevailed at all temperatures used. A 3D model of native α-crystallin with resolution of ~2 nm was created. The model is asymmetrical, has an elongated bean-like shape 13 × 19 nm with a dense core and filamentous "kernel". It does not contain a central cavity. The majority of α-crystallin particles regardless of experimental conditions are 13 × 19 nm, which corresponds to 22S sedimentation coefficient, hydrodynamic diameter 20 nm and mass of 750-830 kD. These particles are in dynamic equilibrium with particles of smaller and larger sizes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7969.map.gz emd_7969.map.gz | 263.6 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7969-v30.xml emd-7969-v30.xml emd-7969.xml emd-7969.xml | 11.2 KB 11.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_7969.png emd_7969.png | 101.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7969 http://ftp.pdbj.org/pub/emdb/structures/EMD-7969 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7969 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7969 | HTTPS FTP |
-Validation report
| Summary document |  emd_7969_validation.pdf.gz emd_7969_validation.pdf.gz | 78.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_7969_full_validation.pdf.gz emd_7969_full_validation.pdf.gz | 77.8 KB | Display | |
| Data in XML |  emd_7969_validation.xml.gz emd_7969_validation.xml.gz | 493 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7969 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7969 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7969 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7969 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_7969.map.gz / Format: CCP4 / Size: 2.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7969.map.gz / Format: CCP4 / Size: 2.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Native alpha-crystallin from bovine eye lens. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.3 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
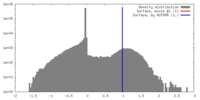
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Native alpha-crystallin from bovine eye lens.
| Entire | Name: Native alpha-crystallin from bovine eye lens. |
|---|---|
| Components |
|
-Supramolecule #1: Native alpha-crystallin from bovine eye lens.
| Supramolecule | Name: Native alpha-crystallin from bovine eye lens. / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 830 KDa |
-Macromolecule #1: Alpha-crystallin A chain CRYAA
| Macromolecule | Name: Alpha-crystallin A chain CRYAA / type: protein_or_peptide / ID: 1 / Details: Alpha-crystallin A chain CRYAA / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: MDIAIQHPWF KRTLGPFYP S RLFDQFFG EG LFEYDLL PFL SSTISP YYRQSLFRTV LDSG ISEVR SDRDK FVIF LDVKHF SPE DLTVKVQ ED FVEIHGKHNE RQDDHGYI S REFHRRYRL PSNVDQSALS CSLSADGML T FSGPKIPS GV DAGHSER AIP VSREEK PSSA PSS |
-Macromolecule #2: Alpha-crystallin B chain CRYAB
| Macromolecule | Name: Alpha-crystallin B chain CRYAB / type: protein_or_peptide / ID: 2 / Details: Alpha-crystallin B chain CRYAB / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: MDIAIHHPWI RRPFFPFHS P SRLFDQFF GE HLLESDL FPA STSLSP FYLRPPSFLR APSW IDTGL SEMRL EKDR FSVNLD VKH FSPEELK VK VLGDVIEVHG KHEERQDE H GFISREFHR KYRIPADVDP LAITSSLSS D GVLTVNGP RK QASGPER TIP ITREEK PAVT AAPKK |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 20 mM Tris-HCl pH7.5, 100 mM NaCl, 1 mM EDTA |
| Staining | Type: NEGATIVE / Material: urany acetate / Details: Valentine and double-carbon |
| Grid | Model: Homemade / Material: COPPER / Mesh: 150 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Details | stored for 48 hr at 4oC prior usage |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 1200EX |
|---|---|
| Image recording | Film or detector model: GATAN MULTISCAN / Average electron dose: 100.0 e/Å2 |
| Electron beam | Acceleration voltage: 100 kV / Electron source: TUNGSTEN HAIRPIN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 1.9 mm |
| Sample stage | Specimen holder model: JEOL |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)