+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6701 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 3D cryo-EM reconstruction of Microtubule-WHAMM complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | microtubule / helical reconstruction / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationplasma membrane tubulation / Arp2/3 complex-mediated actin nucleation / Arp2/3 complex binding / positive regulation of actin nucleation / focal adhesion assembly / RHOD GTPase cycle / lamellipodium assembly / endoplasmic reticulum to Golgi vesicle-mediated transport / endoplasmic reticulum-Golgi intermediate compartment membrane / actin filament organization ...plasma membrane tubulation / Arp2/3 complex-mediated actin nucleation / Arp2/3 complex binding / positive regulation of actin nucleation / focal adhesion assembly / RHOD GTPase cycle / lamellipodium assembly / endoplasmic reticulum to Golgi vesicle-mediated transport / endoplasmic reticulum-Golgi intermediate compartment membrane / actin filament organization / cytoplasmic vesicle membrane / small GTPase binding / actin binding / microtubule binding / microtubule / Golgi membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 4.5 Å | |||||||||
 Authors Authors | Liu T / Wang HW | |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2017 Journal: J Mol Biol / Year: 2017Title: Structural Insights of WHAMM's Interaction with Microtubules by Cryo-EM. Authors: Tianyang Liu / Anbang Dai / Yong Cao / Rui Zhang / Meng-Qiu Dong / Hong-Wei Wang /   Abstract: WASP homolog associated with actin, membranes, and microtubules (WHAMM) is a vertebrate protein functioning in membrane tubulation for intracellular membrane trafficking and specific organelle ...WASP homolog associated with actin, membranes, and microtubules (WHAMM) is a vertebrate protein functioning in membrane tubulation for intracellular membrane trafficking and specific organelle formation. Composed of multiple domains, WHAMM can bind to membrane and microtubule (MT) and promote actin polymerization nucleation. Previous work revealed that WHAMM's activity to promote actin nucleation is repressed upon binding to MTs. Here, we discovered that WHAMM interacts with αβ-tubulin through a small peptide motif within its MT-binding domain. We reconstructed a high-resolution structure of WHAMM's MT-binding motif (MBM) assembling around MTs using cryo-electron microscopy and verified it with chemical cross-linking and mass spectrometry analysis. We also detected a conformational switch of this motif between the non-MT-bound state and the MT-bound state. These discoveries provide new insights into the mechanism by which WHAMM coordinates actin and MT networks, the two major cytoskeletal systems involved in membrane trafficking and membrane remodeling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6701.map.gz emd_6701.map.gz | 141.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6701-v30.xml emd-6701-v30.xml emd-6701.xml emd-6701.xml | 8.6 KB 8.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_6701.png emd_6701.png | 298.9 KB | ||
| Filedesc metadata |  emd-6701.cif.gz emd-6701.cif.gz | 4.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6701 http://ftp.pdbj.org/pub/emdb/structures/EMD-6701 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6701 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6701 | HTTPS FTP |
-Related structure data
| Related structure data |  5x1gMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_6701.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6701.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.306 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
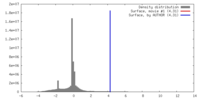
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Ternary complex of alpha,beta-tubulin dimer with microtubule bind...
| Entire | Name: Ternary complex of alpha,beta-tubulin dimer with microtubule binding motif of WHAMM |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of alpha,beta-tubulin dimer with microtubule bind...
| Supramolecule | Name: Ternary complex of alpha,beta-tubulin dimer with microtubule binding motif of WHAMM type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: WASP homolog-associated protein with actin, membranes and microtubules
| Macromolecule | Name: WASP homolog-associated protein with actin, membranes and microtubules type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 4.414182 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: IQMKRDKIKE EEQKKKEWIN QERQKTLQRL RSFK UniProtKB: WASP homolog-associated protein with actin, membranes and microtubules |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 6.8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 8.6 Å Applied symmetry - Helical parameters - Δ&Phi: -25.762 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 4.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 8000 |
|---|---|
| Startup model | Type of model: OTHER |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)