+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6624 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | HIV-1 CSL envelope glycoprotein trimer bound to bnAb PGV04 | |||||||||
 Map data Map data | HIV-1 CSL envelope glycoprotein trimer bound to bnAb PGV04 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | HIV / Envelope / glycoprotein / trimer | |||||||||
| Biological species |   Human immunodeficiency virus / Human immunodeficiency virus /  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 20.0 Å | |||||||||
 Authors Authors | Kong L / He L / de Val N / Morris CD / Vora N / Azadnia P / Sok D / Zhou B / Burton DR / Ward AB ...Kong L / He L / de Val N / Morris CD / Vora N / Azadnia P / Sok D / Zhou B / Burton DR / Ward AB / Wilson IA / Zhu J | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2016 Journal: Nat Commun / Year: 2016Title: Uncleaved prefusion-optimized gp140 trimers derived from analysis of HIV-1 envelope metastability. Authors: Leopold Kong / Linling He / Natalia de Val / Nemil Vora / Charles D Morris / Parisa Azadnia / Devin Sok / Bin Zhou / Dennis R Burton / Andrew B Ward / Ian A Wilson / Jiang Zhu /  Abstract: The trimeric HIV-1 envelope glycoprotein (Env) is critical for host immune recognition and neutralization. Despite advances in trimer design, the roots of Env trimer metastability remain elusive. ...The trimeric HIV-1 envelope glycoprotein (Env) is critical for host immune recognition and neutralization. Despite advances in trimer design, the roots of Env trimer metastability remain elusive. Here we investigate the contribution of two Env regions to metastability. First, we computationally redesign a largely disordered bend in heptad region 1 (HR1) of SOSIP trimers that connects the long, central HR1 helix to the fusion peptide, substantially improving the yield of soluble, well-folded trimers. Structural and antigenic analyses of two distinct HR1 redesigns confirm that redesigned Env closely mimics the native, prefusion trimer with a more stable gp41. Next, we replace the cleavage site between gp120 and gp41 with various linkers in the context of an HR1 redesign. Electron microscopy reveals a potential fusion intermediate state for uncleaved trimers containing short but not long linkers. Together, these results outline a general approach for stabilization of Env trimers from diverse HIV-1 strains. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6624.map.gz emd_6624.map.gz | 1.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6624-v30.xml emd-6624-v30.xml emd-6624.xml emd-6624.xml | 10.5 KB 10.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_6624.tif emd_6624.tif | 33.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6624 http://ftp.pdbj.org/pub/emdb/structures/EMD-6624 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6624 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6624 | HTTPS FTP |
-Validation report
| Summary document |  emd_6624_validation.pdf.gz emd_6624_validation.pdf.gz | 78.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_6624_full_validation.pdf.gz emd_6624_full_validation.pdf.gz | 77.6 KB | Display | |
| Data in XML |  emd_6624_validation.xml.gz emd_6624_validation.xml.gz | 494 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6624 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6624 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6624 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6624 | HTTPS FTP |
-Related structure data
| Related structure data |  6590C 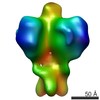 6591C  6592C  6593C  6621C  6622C  6623C  6625C  6626C  6627C  6628C  5js9C  5jsaC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_6624.map.gz / Format: CCP4 / Size: 1.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6624.map.gz / Format: CCP4 / Size: 1.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HIV-1 CSL envelope glycoprotein trimer bound to bnAb PGV04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : HIV-1 CSL envelope glycoprotein trimer bound to PGV04
| Entire | Name: HIV-1 CSL envelope glycoprotein trimer bound to PGV04 |
|---|---|
| Components |
|
-Supramolecule #1000: HIV-1 CSL envelope glycoprotein trimer bound to PGV04
| Supramolecule | Name: HIV-1 CSL envelope glycoprotein trimer bound to PGV04 / type: sample / ID: 1000 / Oligomeric state: trimer / Number unique components: 2 |
|---|---|
| Molecular weight | Theoretical: 570 KDa |
-Macromolecule #1: CSL
| Macromolecule | Name: CSL / type: protein_or_peptide / ID: 1 Details: Heterodimer of gp120 and gp41 assembles into a trimer. Oligomeric state: trimer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus / synonym: HIV Human immunodeficiency virus / synonym: HIV |
| Molecular weight | Theoretical: 420 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: HEK293F Homo sapiens (human) / Recombinant cell: HEK293F |
-Macromolecule #2: bnAb PGV04
| Macromolecule | Name: bnAb PGV04 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / synonym: human Homo sapiens (human) / synonym: human |
| Molecular weight | Theoretical: 50 KDa |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.02 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: 50 mM Tris-HCl, 150 mM NaCl |
| Staining | Type: NEGATIVE Details: Grids were glow discharged at 20 mA for 30 seconds, then stained for 40 seconds using 2% uranyl formate. |
| Grid | Details: 400 Cu mesh grids |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI SPIRIT |
|---|---|
| Date | Oct 23, 2015 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Number real images: 111 / Average electron dose: 30.18 e/Å2 |
| Tilt angle min | 0 |
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Calibrated magnification: 52000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 46000 |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC / Tilt angle max: 50 |
| Experimental equipment |  Model: Tecnai Spirit / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 20.0 Å / Resolution method: OTHER / Software - Name: EMAN2, sparx / Number images used: 12473 |
|---|---|
| Final two d classification | Number classes: 112 |
 Movie
Movie Controller
Controller



 UCSF Chimera
UCSF Chimera




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















