[English] 日本語
 Yorodumi
Yorodumi- EMDB-61520: Class 3 state of the GfsA KSQ-ancestralAT chimeric didomain in co... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Class 3 state of the GfsA KSQ-ancestralAT chimeric didomain in complex with the GfsA ACP domain | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | decarboxylase / polyketide synthase / LYASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationLyases; Carbon-carbon lyases; Carboxy-lyases / macrolide biosynthetic process / fatty acid synthase activity / phosphopantetheine binding / 3-oxoacyl-[acyl-carrier-protein] synthase activity / Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / fatty acid biosynthetic process / lyase activity Similarity search - Function | |||||||||
| Biological species |  Streptomyces graminofaciens (bacteria) Streptomyces graminofaciens (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.71 Å | |||||||||
 Authors Authors | Chisuga T / Liao Z / Adachi N / Kawasaki M / Moriya T / Senda T / Kudo F / Eguchi T / Miyanaga A | |||||||||
| Funding support |  Japan, 2 items Japan, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Ancestral sequence reconstruction as a tool for structural analysis of modular polyketide synthases. Authors: Taichi Chisuga / Shota Takinami / Zengwei Liao / Masayuki Karasawa / Naruhiko Adachi / Masato Kawasaki / Toshio Moriya / Toshiya Senda / Tohru Terada / Fumitaka Kudo / Tadashi Eguchi / Shogo ...Authors: Taichi Chisuga / Shota Takinami / Zengwei Liao / Masayuki Karasawa / Naruhiko Adachi / Masato Kawasaki / Toshio Moriya / Toshiya Senda / Tohru Terada / Fumitaka Kudo / Tadashi Eguchi / Shogo Nakano / Sohei Ito / Akimasa Miyanaga /   Abstract: Modular polyketide synthases (PKSs) are large multi-domain enzymes critical for the biosynthesis of polyketide antibiotics. However, challenges with structural analysis limits our mechanistic ...Modular polyketide synthases (PKSs) are large multi-domain enzymes critical for the biosynthesis of polyketide antibiotics. However, challenges with structural analysis limits our mechanistic understanding of modular PKSs. In this report, we explore the potential of ancestral sequence reconstruction (ASR) for structure analysis of target proteins. As a model, we focus on the FD-891 PKS loading module composed of ketosynthase-like decarboxylase (KS), acyltransferase (AT) and acyl carrier protein (ACP) domains. We construct a KSAncAT chimeric didomain by replacing the native AT with an ancestral AT (AncAT) using ASR. After confirming that KSAncAT chimeric didomain retains similar enzymatic function to the native KSAT didomain, we successfully determine a high-resolution crystal structure of the KSAncAT chimeric didomain and cryo-EM structures of the KS-ACP complex. These cryo-EM structures, which could not be determined for the native protein, exemplify the utility of ASR to enable cryo-EM single-particle analysis. Our findings demonstrate that integrating ASR with structural analysis provides deeper mechanistic insight into modular PKSs. Furthermore, applying ASR to a partial region of the targeted multi-domain proteins could expand the potential of ASR and may serve as a valuable framework for investigating the structure and function of various multi-domain proteins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_61520.map.gz emd_61520.map.gz | 483.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-61520-v30.xml emd-61520-v30.xml emd-61520.xml emd-61520.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_61520.png emd_61520.png | 64.5 KB | ||
| Masks |  emd_61520_msk_1.map emd_61520_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-61520.cif.gz emd-61520.cif.gz | 6.5 KB | ||
| Others |  emd_61520_additional_1.map.gz emd_61520_additional_1.map.gz emd_61520_half_map_1.map.gz emd_61520_half_map_1.map.gz emd_61520_half_map_2.map.gz emd_61520_half_map_2.map.gz | 446.9 MB 475.6 MB 475.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-61520 http://ftp.pdbj.org/pub/emdb/structures/EMD-61520 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-61520 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-61520 | HTTPS FTP |
-Related structure data
| Related structure data |  9jj9MC  9iywC  9jjbC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_61520.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_61520.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.58 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_61520_msk_1.map emd_61520_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_61520_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_61520_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_61520_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Class 3 state of the GfsA KSQ-ancestralAT chimeric didomain in co...
| Entire | Name: Class 3 state of the GfsA KSQ-ancestralAT chimeric didomain in complex with the GfsA ACP domain |
|---|---|
| Components |
|
-Supramolecule #1: Class 3 state of the GfsA KSQ-ancestralAT chimeric didomain in co...
| Supramolecule | Name: Class 3 state of the GfsA KSQ-ancestralAT chimeric didomain in complex with the GfsA ACP domain type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Streptomyces graminofaciens (bacteria) Streptomyces graminofaciens (bacteria) |
-Macromolecule #1: Polyketide synthase GfsA
| Macromolecule | Name: Polyketide synthase GfsA / type: protein_or_peptide / ID: 1 / Details: GfsA KSQ-ancestralAT / Number of copies: 2 / Enantiomer: LEVO EC number: Transferases; Acyltransferases; Transferring groups other than aminoacyl groups |
|---|---|
| Source (natural) | Organism:  Streptomyces graminofaciens (bacteria) Streptomyces graminofaciens (bacteria) |
| Molecular weight | Theoretical: 63.183352 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HMGRSQNSEF ETASDEPIAV IGLSCRLPKA SGPQELWQLL DDGASAVTRV PADRETPPST EEESADGEAA GARWGGFLDR VDTFDAGFF GISPREAAAM DPQQRLVLEL SWEALEGAGL VPATLRDTGL GVFVGAARDD YATLYRRREG RAVDHHAMTG L HRSLIANR ...String: HMGRSQNSEF ETASDEPIAV IGLSCRLPKA SGPQELWQLL DDGASAVTRV PADRETPPST EEESADGEAA GARWGGFLDR VDTFDAGFF GISPREAAAM DPQQRLVLEL SWEALEGAGL VPATLRDTGL GVFVGAARDD YATLYRRREG RAVDHHAMTG L HRSLIANR ISYALGAHGP SMVVDTGCSS SLVAVHLACE SLRRGESDIA LAGGVNLNIA AESARETAAF GGLSPDGQCF TF DARANGF VRGEGGGLVV LKTLRRALAD GDLVHGVILA SAVNNDGPSD TLTTPSRRAQ ESLLTRVYRR AGVTPTEVGY VEL HGTGTK VGDPIEAAAL GAVLGTGRDT PLPVGSIKTN IGHLEGAAGI AGLIKALLQL RRRRLVPSLN FSTPNPDIPL DALN LRVQQ ESAPWATPSG GGRTLVAGVS SFGMGGTNCH VVVSAAPVPE DGETTSEAGA TGPDSGPALL PWVVSARSPQ ALRDQ AGRL AAWADSPAGR EASPVDIGWS LATSRTHFEY RAVVSGSDRD ELVASLRALA SGSPVTAAGA VDGRAEPVAL LSAVGE LFA DGYPVDWTAY FAGWPAARVE LPTYAFQRSR HWLENVPELA VS UniProtKB: Polyketide synthase GfsA, Polyketide synthase GfsA |
-Macromolecule #2: Polyketide synthase
| Macromolecule | Name: Polyketide synthase / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Streptomyces graminofaciens (bacteria) Streptomyces graminofaciens (bacteria) |
| Molecular weight | Theoretical: 10.244458 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HMPREPVTPD SDHPDPVETV RQLTAHVLGL TAAADVEMTR SFKDLGFDSL MSVELRDRLC AATGLSLATT LLYDHPSPAE TAEFVRARL TGDEA UniProtKB: Polyketide synthase GfsA |
-Macromolecule #3: N-[2-(acetylamino)ethyl]-N~3~-[(2R)-2-hydroxy-3,3-dimethyl-4-(pho...
| Macromolecule | Name: N-[2-(acetylamino)ethyl]-N~3~-[(2R)-2-hydroxy-3,3-dimethyl-4-(phosphonooxy)butanoyl]-beta-alaninamide type: ligand / ID: 3 / Number of copies: 1 / Formula: 9EF |
|---|---|
| Molecular weight | Theoretical: 383.335 Da |
| Chemical component information | 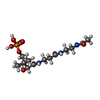 ChemComp-9EF: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.0 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 291 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















































