+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5688 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structural insight into the thermoTRPV channels assembly | |||||||||
 Map data Map data | TRPV2 structure at 13.6 A | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TRP channel | |||||||||
| Function / homology |  Function and homology information Function and homology informationplasma membrane => GO:0005886 / growth cone membrane / TRP channels / response to temperature stimulus / positive regulation of calcium ion import / calcium ion import across plasma membrane / axonal growth cone / positive regulation of axon extension / monoatomic cation channel activity / endomembrane system ...plasma membrane => GO:0005886 / growth cone membrane / TRP channels / response to temperature stimulus / positive regulation of calcium ion import / calcium ion import across plasma membrane / axonal growth cone / positive regulation of axon extension / monoatomic cation channel activity / endomembrane system / calcium channel activity / melanosome / lamellipodium / positive regulation of cold-induced thermogenesis / cell body / axon / negative regulation of cell population proliferation / cell surface / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 13.6 Å | |||||||||
 Authors Authors | Huynh KW / Cohen MR / Chakrapani S / Holdaway HA / Stewart PL / Moiseenkova-Bell VY | |||||||||
 Citation Citation |  Journal: Structure / Year: 2014 Journal: Structure / Year: 2014Title: Structural insight into the assembly of TRPV channels. Authors: Kevin W Huynh / Matthew R Cohen / Sudha Chakrapani / Heather A Holdaway / Phoebe L Stewart / Vera Y Moiseenkova-Bell /  Abstract: Transient receptor potential (TRP) proteins are a large family of polymodal nonselective cation channels. The TRP vanilloid (TRPV) subfamily consists of six homologous members with diverse functions. ...Transient receptor potential (TRP) proteins are a large family of polymodal nonselective cation channels. The TRP vanilloid (TRPV) subfamily consists of six homologous members with diverse functions. TRPV1-TRPV4 are nonselective cation channels proposed to play a role in nociception, while TRPV5 and TRPV6 are involved in epithelial Ca²⁺ homeostasis. Here we present the cryo-electron microscopy (cryo-EM) structure of functional, full-length TRPV2 at 13.6 Å resolution. The map reveals that the TRPV2 cytoplasmic domain displays a 4-fold petal-like shape in which high-resolution N-terminal ankyrin repeat domain (ARD) structures can be unambiguously fitted. Fitting of the available ARD structures for other TRPV subfamily members into the TRPV2 EM map suggests that TRPV subfamily members have highly homologous structural topologies. These results allowed us to postulate a structural explanation for the functional diversity among TRPV channels and their differential regulation by proteins and ligands. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5688.map.gz emd_5688.map.gz | 10.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5688-v30.xml emd-5688-v30.xml emd-5688.xml emd-5688.xml | 10.5 KB 10.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5688.png emd_5688.png | 46.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5688 http://ftp.pdbj.org/pub/emdb/structures/EMD-5688 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5688 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5688 | HTTPS FTP |
-Validation report
| Summary document |  emd_5688_validation.pdf.gz emd_5688_validation.pdf.gz | 78.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_5688_full_validation.pdf.gz emd_5688_full_validation.pdf.gz | 77.4 KB | Display | |
| Data in XML |  emd_5688_validation.xml.gz emd_5688_validation.xml.gz | 494 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5688 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5688 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5688 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5688 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_5688.map.gz / Format: CCP4 / Size: 15.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5688.map.gz / Format: CCP4 / Size: 15.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TRPV2 structure at 13.6 A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.66 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
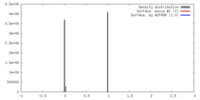
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Rat Transient Receptor Potential Vanilloid 2 (TRPV2)
| Entire | Name: Rat Transient Receptor Potential Vanilloid 2 (TRPV2) |
|---|---|
| Components |
|
-Supramolecule #1000: Rat Transient Receptor Potential Vanilloid 2 (TRPV2)
| Supramolecule | Name: Rat Transient Receptor Potential Vanilloid 2 (TRPV2) / type: sample / ID: 1000 / Details: sample was monodisperse / Oligomeric state: tetramer / Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 600 KDa / Theoretical: 360 KDa / Method: Size-exclusion chromatography |
-Macromolecule #1: Transient Receptor Potential Vanilloid 2
| Macromolecule | Name: Transient Receptor Potential Vanilloid 2 / type: protein_or_peptide / ID: 1 / Name.synonym: TRPV2 / Number of copies: 1 / Oligomeric state: tetramer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 600 KDa / Theoretical: 300 KDa |
| Recombinant expression | Organism:  |
| Sequence | UniProtKB: Transient receptor potential cation channel subfamily V member 2 GO: plasma membrane => GO:0005886 InterPro: Transient receptor potential cation channel subfamily V |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.5 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 20 mM HEPES, 150 mM NaCl, 1.0mM DTT, 0.006% DMNG |
| Grid | Details: 400 mesh Quantifoil R2/1 copper grids |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 120 K / Instrument: FEI VITROBOT MARK IV / Method: blot for 1, 2, or 3 sec |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Temperature | Min: 80 K / Max: 105 K / Average: 100 K |
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 200,000 times magnification |
| Date | Oct 17, 2012 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Number real images: 650 / Average electron dose: 10 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 117293 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.12 mm / Nominal defocus max: -3.0 µm / Nominal defocus min: -1.5 µm / Nominal magnification: 62000 |
| Sample stage | Specimen holder: 626 / Specimen holder model: GATAN LIQUID NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | EMAN2 |
|---|---|
| CTF correction | Details: each image |
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 13.6 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: Imagic, FREALIGN / Number images used: 23051 |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)