[English] 日本語
 Yorodumi
Yorodumi- EMDB-5453: A Tail-like Assembly at the Portal Vertex in Intact Herpes Simple... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5453 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | A Tail-like Assembly at the Portal Vertex in Intact Herpes Simplex Type-1 Virions | |||||||||
 Map data Map data | Reconstruction of HSV-1 virion using Melon Ball alignment | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | HSV-1 / cryo-ET / cryo-EM / Electron cryo-microscopy / Electron cryo-tomography / PVAT / MSA / Melon Ball / Single Particle Tomography / Sub-tomogram Averaging | |||||||||
| Biological species |   Human herpesvirus 1 (Herpes simplex virus type 1) Human herpesvirus 1 (Herpes simplex virus type 1) | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 60.0 Å | |||||||||
 Authors Authors | Schmid MF / Hecksel CW / Rochat RH / Bhella D / Chiu W / Rixon FJ | |||||||||
 Citation Citation |  Journal: PLoS Pathog / Year: 2012 Journal: PLoS Pathog / Year: 2012Title: A tail-like assembly at the portal vertex in intact herpes simplex type-1 virions. Authors: Michael F Schmid / Corey W Hecksel / Ryan H Rochat / David Bhella / Wah Chiu / Frazer J Rixon /  Abstract: Herpes viruses are prevalent and well characterized human pathogens. Despite extensive study, much remains to be learned about the structure of the genome packaging and release machinery in the ...Herpes viruses are prevalent and well characterized human pathogens. Despite extensive study, much remains to be learned about the structure of the genome packaging and release machinery in the capsids of these large and complex double-stranded DNA viruses. However, such machinery is well characterized in tailed bacteriophage, which share a common evolutionary origin with herpesvirus. In tailed bacteriophage, the genome exits from the virus particle through a portal and is transferred into the host cell by a complex apparatus (i.e. the tail) located at the portal vertex. Here we use electron cryo-tomography of human herpes simplex type-1 (HSV-1) virions to reveal a previously unsuspected feature at the portal vertex, which extends across the HSV-1 tegument layer to form a connection between the capsid and the viral membrane. The location of this assembly suggests that it plays a role in genome release into the nucleus and is also important for virion architecture. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5453.map.gz emd_5453.map.gz | 125.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5453-v30.xml emd-5453-v30.xml emd-5453.xml emd-5453.xml | 11 KB 11 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5453_1.jpg emd_5453_1.jpg | 65.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5453 http://ftp.pdbj.org/pub/emdb/structures/EMD-5453 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5453 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5453 | HTTPS FTP |
-Validation report
| Summary document |  emd_5453_validation.pdf.gz emd_5453_validation.pdf.gz | 78.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_5453_full_validation.pdf.gz emd_5453_full_validation.pdf.gz | 77.2 KB | Display | |
| Data in XML |  emd_5453_validation.xml.gz emd_5453_validation.xml.gz | 494 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5453 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5453 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5453 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5453 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5453.map.gz / Format: CCP4 / Size: 159.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5453.map.gz / Format: CCP4 / Size: 159.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of HSV-1 virion using Melon Ball alignment | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 10.8 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
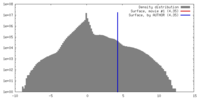
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Herpes Simplex Virus type 1 (HSV-1) virion.
| Entire | Name: Herpes Simplex Virus type 1 (HSV-1) virion. |
|---|---|
| Components |
|
-Supramolecule #1000: Herpes Simplex Virus type 1 (HSV-1) virion.
| Supramolecule | Name: Herpes Simplex Virus type 1 (HSV-1) virion. / type: sample / ID: 1000 / Details: Frozen hydrated HSV-1 Virions / Oligomeric state: 1 / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 200 MDa |
-Supramolecule #1: Human herpesvirus 1
| Supramolecule | Name: Human herpesvirus 1 / type: virus / ID: 1 / Name.synonym: Herpes Simplex Virus type 1 / Details: Frozen hydrated virions / NCBI-ID: 10298 / Sci species name: Human herpesvirus 1 / Database: NCBI / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: No / Syn species name: Herpes Simplex Virus type 1 |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) / synonym: VERTEBRATES Homo sapiens (human) / synonym: VERTEBRATES |
| Molecular weight | Theoretical: 200 MDa |
| Virus shell | Shell ID: 1 / Diameter: 1250 Å / T number (triangulation number): 16 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.2 Details: 170mM NaCl, 3.4mM KCl, 10mM Na2HPO4, 1.8mM KH2PO4, 4.9mM MgCl2, 6.8mM CaCl2 |
|---|---|
| Grid | Details: Quantifoil R 2/2 holey carbon film on a 200 mesh copper grid, glow discharged in air |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 120 K / Instrument: FEI VITROBOT MARK IV / Method: Manual blotting |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 2200FS |
|---|---|
| Temperature | Average: 100 K |
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 100,000 times magnification |
| Specialist optics | Energy filter - Name: Omega / Energy filter - Lower energy threshold: 25.0 eV / Energy filter - Upper energy threshold: 30.0 eV |
| Date | May 23, 2008 |
| Image recording | Category: FILM / Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Digitization - Scanner: OTHER / Digitization - Sampling interval: 15 µm / Number real images: 11 / Average electron dose: 88 e/Å2 / Bits/pixel: 16 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 28195 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 4.0 µm / Nominal magnification: 20000 |
| Sample stage | Specimen holder: Gatan 914 / Specimen holder model: GATAN LIQUID NITROGEN / Tilt series - Axis1 - Min angle: -60 ° / Tilt series - Axis1 - Max angle: 60 ° |
- Image processing
Image processing
| Details | Multivariate Statistical Analysis (MSA) was used to classify a unique vertex in the capsid shell for alignment and averaging. Further details provided in manuscript. Average number of projections used in the 3D reconstructions: 214. |
|---|---|
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 60.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: IMOD, tomohunter / Details: Final map is the average of 214 subvolumes |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)