[English] 日本語
 Yorodumi
Yorodumi- EMDB-53065: Respiratory supercomplex from Mycobacterium smegmatis with decylu... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Respiratory supercomplex from Mycobacterium smegmatis with decylubiquinone | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RESPIRATORY SUPERCOMPLEX / MEMBRANE PROTEIN / ACTINOBACTERIA / ELECTRON TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationaerobic electron transport chain / cytochrome-c oxidase / oxidative phosphorylation / quinol-cytochrome-c reductase / quinol-cytochrome-c reductase activity / cytochrome-c oxidase activity / superoxide dismutase / superoxide dismutase activity / electron transport coupled proton transport / oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen ...aerobic electron transport chain / cytochrome-c oxidase / oxidative phosphorylation / quinol-cytochrome-c reductase / quinol-cytochrome-c reductase activity / cytochrome-c oxidase activity / superoxide dismutase / superoxide dismutase activity / electron transport coupled proton transport / oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen / ATP synthesis coupled electron transport / respiratory electron transport chain / monooxygenase activity / electron transport chain / 2 iron, 2 sulfur cluster binding / iron ion binding / copper ion binding / heme binding / metal ion binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Kovalova T / Krol S / Brzezinski P / Hogbom M | |||||||||
| Funding support |  Sweden, 1 items Sweden, 1 items
| |||||||||
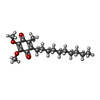
 Citation Citation |  Journal: Commun Biol / Year: 2025 Journal: Commun Biol / Year: 2025Title: Mycobacterial respiratory chain enzymes and growth are inhibited by decylubiquinone. Authors: Sylwia Król / Terezia Kovalova / Mateusz Janczak / Sadaf Kalsum / Mira Akber / Martin Högbom / Susanna Brighenti / Pia Ädelroth / Peter Brzezinski /  Abstract: Aerobic organisms obtain energy by linking electron transfer from NADH to O, through the respiratory chain, to transmembrane proton translocation. In mycobacteria the respiratory chain is branched; ...Aerobic organisms obtain energy by linking electron transfer from NADH to O, through the respiratory chain, to transmembrane proton translocation. In mycobacteria the respiratory chain is branched; the membrane-bound electron carrier menaquinol (MQH) donates electrons either to the O-reducing cytochrome bd or a supercomplex that is composed of a complex (C) III dimer flanked by two CIVs. Here, we measured the dimethyl-naphthoquinone (DMNQH a menaquinol analogue) oxidation:O reduction activities of the CIIICIV supercomplex and cytochrome bd in the presence of an analogue (decylubiquinone, DCQ) of the mammalian electron carrier, ubiquinol. The data show that DCQH inhibits both the CIIICIV and cytochrome bd activities, suggesting that DCQ/DCQH interferes with both branches of the respiratory chain. Cryo-EM data of the M. smegmatis supercomplex shows that oxidized DCQ binds in the electron donor site (Q) of CIII. Accordingly, growth of M. smegmatis cells was impaired in the presence of DCQ. Remarkably, DCQ also impairs intracellular growth of virulent M. tuberculosis cells in human primary macrophages suggesting that the compound could potentially be used as an adjuvant during tuberculosis disease treatment. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_53065.map.gz emd_53065.map.gz | 300.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-53065-v30.xml emd-53065-v30.xml emd-53065.xml emd-53065.xml | 42.5 KB 42.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_53065.png emd_53065.png | 84.8 KB | ||
| Filedesc metadata |  emd-53065.cif.gz emd-53065.cif.gz | 10.1 KB | ||
| Others |  emd_53065_additional_1.map.gz emd_53065_additional_1.map.gz emd_53065_half_map_1.map.gz emd_53065_half_map_1.map.gz emd_53065_half_map_2.map.gz emd_53065_half_map_2.map.gz | 567.1 MB 556.7 MB 556.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-53065 http://ftp.pdbj.org/pub/emdb/structures/EMD-53065 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-53065 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-53065 | HTTPS FTP |
-Validation report
| Summary document |  emd_53065_validation.pdf.gz emd_53065_validation.pdf.gz | 1.3 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_53065_full_validation.pdf.gz emd_53065_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  emd_53065_validation.xml.gz emd_53065_validation.xml.gz | 19.7 KB | Display | |
| Data in CIF |  emd_53065_validation.cif.gz emd_53065_validation.cif.gz | 23.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-53065 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-53065 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-53065 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-53065 | HTTPS FTP |
-Related structure data
| Related structure data |  9qefMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_53065.map.gz / Format: CCP4 / Size: 600.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_53065.map.gz / Format: CCP4 / Size: 600.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.825 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_53065_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_53065_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_53065_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : The respiratory supercomplex CIII2CIV2
+Supramolecule #1: The respiratory supercomplex CIII2CIV2
+Macromolecule #1: Cytochrome bc1 complex cytochrome c subunit
+Macromolecule #2: Cytochrome bc1 complex cytochrome c subunit
+Macromolecule #3: Cytochrome bc1 complex cytochrome b subunit
+Macromolecule #4: Transmembrane protein
+Macromolecule #5: Probable cytochrome c oxidase subunit 3
+Macromolecule #6: Cytochrome c oxidase polypeptide 4
+Macromolecule #7: Cytochrome c oxidase subunit 1
+Macromolecule #8: cytochrome-c oxidase
+Macromolecule #9: Cytochrome c oxidase subunit
+Macromolecule #10: Uncharacterized protein MSMEG_4692/MSMEI_4575
+Macromolecule #11: LpqE protein
+Macromolecule #12: Superoxide dismutase [Cu-Zn]
+Macromolecule #13: Co-purified transmembrane protein
+Macromolecule #14: Co-purified peptide
+Macromolecule #15: HEME C
+Macromolecule #16: MENAQUINONE-9
+Macromolecule #17: acyl-phosphatidyl-myo-inositol dimannoside (AcPIM2)
+Macromolecule #18: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #19: [(2~{R})-3-[[(1~{S},2~{R},3~{S},4~{S},5~{R},6~{R})-2-[(2~{R},3~{S...
+Macromolecule #20: (2R)-2-(hexadecanoyloxy)-3-{[(S)-hydroxy{[(1R,2R,3R,4R,5R,6S)-2,3...
+Macromolecule #21: (1R)-2-(dodecanoyloxy)-1-[(phosphonooxy)methyl]ethyl tetradecanoate
+Macromolecule #22: PROTOPORPHYRIN IX CONTAINING FE
+Macromolecule #23: CARDIOLIPIN
+Macromolecule #24: PALMITIC ACID
+Macromolecule #25: (2S)-1-(hexadecanoyloxy)propan-2-yl (10S)-10-methyloctadecanoate
+Macromolecule #26: TRIDECANE
+Macromolecule #27: 1,2-Distearoyl-sn-glycerophosphoethanolamine
+Macromolecule #28: HEME-A
+Macromolecule #29: COPPER (II) ION
+Macromolecule #30: MAGNESIUM ION
+Macromolecule #31: CALCIUM ION
+Macromolecule #32: 2-decyl-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione
+Macromolecule #33: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.6 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)