+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | BmrA E504A in complex with Hoechst33342 | |||||||||
 Map data Map data | sharpened map by cryosparc v4.5 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | transporter complex with Hoechst33342 / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationATPase-coupled lipid transmembrane transporter activity / Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate / ABC-type transporter activity / transmembrane transport / response to antibiotic / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
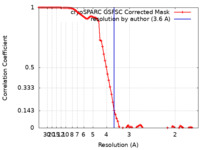
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Moissonnier L / Zarkadas E / Schoehn G / Falson P / Chaptal V | |||||||||
| Funding support |  France, 2 items France, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Rhodamine6G and Hœchst33342 narrow BmrA conformational spectrum for a more efficient use of ATP. Authors: A Gobet / L Moissonnier / E Zarkadas / S Magnard / E Bettler / J Martin / R Terreux / G Schoehn / C Orelle / J M Jault / P Falson / V Chaptal /   Abstract: Multidrug ABC transporters harness the energy of ATP binding and hydrolysis to translocate substrates out of the cell and detoxify them. While this involves a well-accepted alternating access ...Multidrug ABC transporters harness the energy of ATP binding and hydrolysis to translocate substrates out of the cell and detoxify them. While this involves a well-accepted alternating access mechanism, molecular details of this interplay are still elusive. Rhodamine6G binding on a catalytic inactive mutant of the homodimeric multidrug ABC transporter BmrA triggers a cooperative binding of ATP on the two identical nucleotide-binding-sites, otherwise michaelian. Here, we investigate this asymmetric behavior via a structural-enzymology approach, solving cryoEM structures of BmrA at defined ATP ratios, highlighting the plasticity of BmrA as it undergoes the transition from inward to outward facing conformations. Analysis of continuous heterogeneity within cryoEM data and structural dynamics, reveals that Rhodamine6G narrows the conformational spectrum explored by the nucleotide-binding domains. We observe the same behavior for the other drug Hœchst33342. Following on these findings, the effect of drug-binding showed an ATPase stimulation and a maximal transport activity of the wild-type protein at the concentration-range where the cooperative transition occurs. Altogether, these findings provide a description of the influence of drug binding on the ATP-binding sites through a change in conformational dynamics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_51550.map.gz emd_51550.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-51550-v30.xml emd-51550-v30.xml emd-51550.xml emd-51550.xml | 21.6 KB 21.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_51550_fsc.xml emd_51550_fsc.xml | 8.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_51550.png emd_51550.png | 77.3 KB | ||
| Filedesc metadata |  emd-51550.cif.gz emd-51550.cif.gz | 6.5 KB | ||
| Others |  emd_51550_additional_1.map.gz emd_51550_additional_1.map.gz emd_51550_half_map_1.map.gz emd_51550_half_map_1.map.gz emd_51550_half_map_2.map.gz emd_51550_half_map_2.map.gz | 31.6 MB 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-51550 http://ftp.pdbj.org/pub/emdb/structures/EMD-51550 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-51550 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-51550 | HTTPS FTP |
-Validation report
| Summary document |  emd_51550_validation.pdf.gz emd_51550_validation.pdf.gz | 881.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_51550_full_validation.pdf.gz emd_51550_full_validation.pdf.gz | 880.7 KB | Display | |
| Data in XML |  emd_51550_validation.xml.gz emd_51550_validation.xml.gz | 16.3 KB | Display | |
| Data in CIF |  emd_51550_validation.cif.gz emd_51550_validation.cif.gz | 21.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51550 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51550 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51550 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51550 | HTTPS FTP |
-Related structure data
| Related structure data |  9gsjMC  8rezC  8rf1C  8rg7C  8rgaC  8rgnC  8ri1C  8riaC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_51550.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_51550.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map by cryosparc v4.5 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.839 Å | ||||||||||||||||||||||||||||||||||||
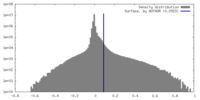
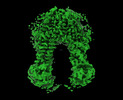
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: un-sharpened map
| File | emd_51550_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | un-sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
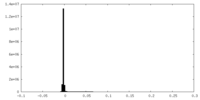
| Density Histograms |
-Half map: Half-map A
| File | emd_51550_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
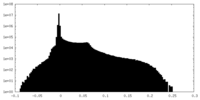
| Density Histograms |
-Half map: Half-map B
| File | emd_51550_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
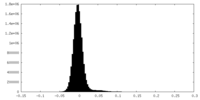
| Density Histograms |
- Sample components
Sample components
-Entire : BmrA E504A in complex with Hoechst33342
| Entire | Name: BmrA E504A in complex with Hoechst33342 |
|---|---|
| Components |
|
-Supramolecule #1: BmrA E504A in complex with Hoechst33342
| Supramolecule | Name: BmrA E504A in complex with Hoechst33342 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 130 KDa |
-Macromolecule #1: Multidrug resistance ABC transporter ATP-binding/permease protein BmrA
| Macromolecule | Name: Multidrug resistance ABC transporter ATP-binding/permease protein BmrA type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 65.747141 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSSSHHHHHH MPTKKQKSKS KLKPFFALVR RTNPSYGKLA FALALSVVTT LVSLLIPLLT KQLVDGFSMS NLSGTQIGLI ALVFFVQAG LSAYATYALN YNGQKIISGL RELLWKKLIK LPVSYFDTNA SGETVSRVTN DTMVVKELIT THISGFITGI I SVIGSLTI ...String: MSSSHHHHHH MPTKKQKSKS KLKPFFALVR RTNPSYGKLA FALALSVVTT LVSLLIPLLT KQLVDGFSMS NLSGTQIGLI ALVFFVQAG LSAYATYALN YNGQKIISGL RELLWKKLIK LPVSYFDTNA SGETVSRVTN DTMVVKELIT THISGFITGI I SVIGSLTI LFIMNWKLTL LVLVVVPLAA LILVPIGRKM FSISRETQDE TARFTGLLNQ ILPEIRLVKA SNAEDVEYGR GK MGISSLF KLGVREAKVQ SLVGPLISLV LMAALVAVIG YGGMQVSSGE LTAGALVAFI LYLFQIIMPM GQITTFFTQL QKS IGATER MIEILAEEEE DTVTGKQIEN AHLPIQLDRV SFGYKPDQLI LKEVSAVIEA GKVTAIVGPS GGGKTTLFKL LERF YSPTA GTIRLGDEPV DTYSLESWRE HIGYVSQESP LMSGTIRENI CYGLERDVTD AEIEKAAEMA YALNFIKELP NQFDT EVGE RGIMLSGGQR QRIAIARALL RNPSILMLDA ATSSLDSQSE KSVQQALEVL MEGRTTIVIA HRLSTVVDAD QLLFVE KGE ITGRGTHHEL MASHGLYRDF AEQQLKMNAD LENKAG UniProtKB: Multidrug resistance ABC transporter ATP-binding/permease protein BmrA |
-Macromolecule #2: 2'-(4-ETHOXYPHENYL)-5-(4-METHYL-1-PIPERAZINYL)-2,5'-BI-BENZIMIDAZOLE
| Macromolecule | Name: 2'-(4-ETHOXYPHENYL)-5-(4-METHYL-1-PIPERAZINYL)-2,5'-BI-BENZIMIDAZOLE type: ligand / ID: 2 / Number of copies: 2 / Formula: HT1 |
|---|---|
| Molecular weight | Theoretical: 452.551 Da |
| Chemical component information |  ChemComp-HT1: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK IV |
| Details | Membrane protein in detergent |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 49.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)