[English] 日本語
 Yorodumi
Yorodumi- EMDB-51522: Cryo-electron microscopy structure of glucose/xylose isomerase fr... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-electron microscopy structure of glucose/xylose isomerase from Streptomyces rubiginosus with magnesium ions in the active site | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | magnesium / isomerase / sugars / glucose / xylose / metal ion / Cryo-EM / METAL BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationxylose isomerase / xylose isomerase activity / D-xylose metabolic process / magnesium ion binding / identical protein binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Streptomyces rubiginosus (bacteria) Streptomyces rubiginosus (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.0 Å | |||||||||
 Authors Authors | Slawek J / Klonecka A / Rawski M / Kozak M | |||||||||
| Funding support |  Poland, 1 items Poland, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-electron microscopy structure of glucose/xylose isomerase from Streptomyces rubiginosus with magnesium ions in the active site Authors: Slawek J / Klonecka A / Rawski M / Kozak M | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_51522.map.gz emd_51522.map.gz | 32.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-51522-v30.xml emd-51522-v30.xml emd-51522.xml emd-51522.xml | 16.1 KB 16.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_51522_fsc.xml emd_51522_fsc.xml | 11.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_51522.png emd_51522.png | 117.1 KB | ||
| Filedesc metadata |  emd-51522.cif.gz emd-51522.cif.gz | 5.6 KB | ||
| Others |  emd_51522_half_map_1.map.gz emd_51522_half_map_1.map.gz emd_51522_half_map_2.map.gz emd_51522_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-51522 http://ftp.pdbj.org/pub/emdb/structures/EMD-51522 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-51522 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-51522 | HTTPS FTP |
-Validation report
| Summary document |  emd_51522_validation.pdf.gz emd_51522_validation.pdf.gz | 782.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_51522_full_validation.pdf.gz emd_51522_full_validation.pdf.gz | 781.9 KB | Display | |
| Data in XML |  emd_51522_validation.xml.gz emd_51522_validation.xml.gz | 15.9 KB | Display | |
| Data in CIF |  emd_51522_validation.cif.gz emd_51522_validation.cif.gz | 21.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51522 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51522 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51522 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-51522 | HTTPS FTP |
-Related structure data
| Related structure data |  9greMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_51522.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_51522.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||
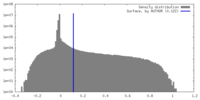
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_51522_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_51522_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : glucose/xylose isomerase with cobalt ions
| Entire | Name: glucose/xylose isomerase with cobalt ions |
|---|---|
| Components |
|
-Supramolecule #1: glucose/xylose isomerase with cobalt ions
| Supramolecule | Name: glucose/xylose isomerase with cobalt ions / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Streptomyces rubiginosus (bacteria) Streptomyces rubiginosus (bacteria) |
| Molecular weight | Theoretical: 43 KDa |
-Macromolecule #1: Xylose isomerase
| Macromolecule | Name: Xylose isomerase / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: xylose isomerase |
|---|---|
| Source (natural) | Organism:  Streptomyces rubiginosus (bacteria) Streptomyces rubiginosus (bacteria) |
| Molecular weight | Theoretical: 43.283297 KDa |
| Sequence | String: MNYQPTPEDR FTFGLWTVGW QGRDPFGDAT RRALDPVESV RRLAELGAHG VTFHDDDLIP FGSSDSEREE HVKRFRQALD DTGMKVPMA TTNLFTHPVF KDGGFTANDR DVRRYALRKT IRNIDLAVEL GAETYVAWGG REGAESGGAK DVRDALDRMK E AFDLLGEY ...String: MNYQPTPEDR FTFGLWTVGW QGRDPFGDAT RRALDPVESV RRLAELGAHG VTFHDDDLIP FGSSDSEREE HVKRFRQALD DTGMKVPMA TTNLFTHPVF KDGGFTANDR DVRRYALRKT IRNIDLAVEL GAETYVAWGG REGAESGGAK DVRDALDRMK E AFDLLGEY VTSQGYDIRF AIEPKPNEPR GDILLPTVGH ALAFIERLER PELYGVNPEV GHEQMAGLNF PHGIAQALWA GK LFHIDLN GQNGIKYDQD LRFGAGDLRA AFWLVDLLES AGYSGPRHFD FKPPRTEDFD GVWASAAGCM RNYLILKERA AAF RADPEV QEALRASRLD ELARPTAADG LQALLDDRSA FEEFDVDAAA ARGMAFERLD QLAMDHLLGA RG UniProtKB: Xylose isomerase |
-Macromolecule #2: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 2 / Number of copies: 8 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #3: water
| Macromolecule | Name: water / type: ligand / ID: 3 / Number of copies: 4 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.9 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Component - Concentration: 50.0 mM / Component - Formula: PBS / Component - Name: Phosphate-buffered saline / Details: 50 mM PBS pH 7.4 |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 5 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)