+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Expanded formalin inactivated CVB1 | |||||||||||||||
 Map data Map data | Density map of expanded formalin inactivated CVB1 resolved at 3 A. | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | coxsackievirus B1 / vaccine / formalin / VIRUS | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont genome entry into host cell via pore formation in plasma membrane / viral capsid / host cell cytoplasm / symbiont-mediated suppression of host gene expression / virion attachment to host cell / structural molecule activity Similarity search - Function | |||||||||||||||
| Biological species |  Coxsackievirus B1 Coxsackievirus B1 | |||||||||||||||
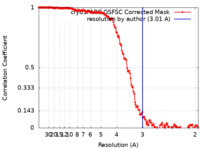
| Method | single particle reconstruction / cryo EM / Resolution: 3.01 Å | |||||||||||||||
 Authors Authors | Plavec Z / Butcher SJ | |||||||||||||||
| Funding support |  Finland, 4 items Finland, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Res Sq / Year: 2024 Journal: Res Sq / Year: 2024Title: Comparison of structure and immunogenicity of CVB1-VLP and inactivated CVB1 vaccine candidates. Authors: Saana Soppela / Zlatka Plavec / Stina Gröhn / Minne Jartti / Sami Oikarinen / Mira Laajala / Varpu Marjomaki / Sarah J Butcher / Minna M Hankaniemi Abstract: Coxsackievirus B1 (CVB1) is a common cause of acute and chronic myocarditis, dilated cardiomyopathy and aseptic meningitis. However, no CVB-vaccines are available for human use. In this study, we ...Coxsackievirus B1 (CVB1) is a common cause of acute and chronic myocarditis, dilated cardiomyopathy and aseptic meningitis. However, no CVB-vaccines are available for human use. In this study, we investigated the immunogenicity of virus-like particle (VLP) and inactivated whole-virus vaccines for CVB1 when administrated to mice via either subcutaneous or intranasal routes formulated with and without commercial and experimental adjuvants. Here, the potential of utilizing epigallocatechin-3-gallate (EGCG) as a mucosal adjuvant synergistically with its ability to inactivate the virus were investigated. EGCG had promising adjuvant properties for CVB1-VLP when administered via the parenteral route but limited efficacy via intranasal administration. However, intranasal administration of the formalin-inactivated virus induced high CVB1-specific humoral, cellular, and mucosal immune responses. Also, based on CVB1-specific IgG-antibody responses, we conclude that CVB1-VLP can be taken up by immune cells when administrated intranasally and further structural engineering for the VLP may increase the mucosal immunogenicity. The preparations contained mixtures of compact and expanded A particles with 85% expanded in the formalin-inactivated virus, but only 52% in the VLP observed by cryogenic electron microscopy. To correlate the structure to immunogenicity, we solved the structures of the CVB1-VLP and the formalin-inactivated CVB1 virus at resolutions ranging from 2.15 A to 4.1 A for the expanded and compact VLP and virus particles by image reconstruction. These structures can be used in designing mutations increasing the stability and immunogenicity of CVB1-VLP in the future. Overall, our results highlight the potential of using formalin inactivated CVB1 vaccine in mucosal immunization programs and provide important information for future development of VLP-based vaccines against all enteroviruses. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_50500.map.gz emd_50500.map.gz | 173.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-50500-v30.xml emd-50500-v30.xml emd-50500.xml emd-50500.xml | 24.9 KB 24.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_50500_fsc.xml emd_50500_fsc.xml | 14.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_50500.png emd_50500.png | 70 KB | ||
| Filedesc metadata |  emd-50500.cif.gz emd-50500.cif.gz | 6.9 KB | ||
| Others |  emd_50500_half_map_1.map.gz emd_50500_half_map_1.map.gz emd_50500_half_map_2.map.gz emd_50500_half_map_2.map.gz | 322.9 MB 322.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-50500 http://ftp.pdbj.org/pub/emdb/structures/EMD-50500 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50500 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50500 | HTTPS FTP |
-Related structure data
| Related structure data |  9fjeMC  9fjcC  9fjdC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_50500.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_50500.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Density map of expanded formalin inactivated CVB1 resolved at 3 A. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.951 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map of expanded formalin inactivated CVB1.
| File | emd_50500_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of expanded formalin inactivated CVB1. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of expanded formalin inactivated CVB1.
| File | emd_50500_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of expanded formalin inactivated CVB1. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Coxsackievirus B1
| Entire | Name:  Coxsackievirus B1 Coxsackievirus B1 |
|---|---|
| Components |
|
-Supramolecule #1: Coxsackievirus B1
| Supramolecule | Name: Coxsackievirus B1 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all Details: Coxsackievirus B1 (CVB1-10796, isolated from Argentina) NCBI-ID: 12071 / Sci species name: Coxsackievirus B1 / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: Yes |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Virus shell | Shell ID: 1 / Name: capsid |
-Macromolecule #1: Capsid protein VP1
| Macromolecule | Name: Capsid protein VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Coxsackievirus B1 Coxsackievirus B1 |
| Molecular weight | Theoretical: 24.943195 KDa |
| Sequence | String: SRSESSIENF LCRAACVYYA TYTNNSEKGY AEWVINTRQV AQLRRKLELF TYLRFDLELT FVITSAQQPS TATSVDAPVQ THQIMYVPP GGPVPTKVTD YAWQTSTNPS VFWTEGNAPP RMSIPFISIG NAYSCFYDGW TQFSRNGVYG INTLNNMGTL Y MRHVNEAG ...String: SRSESSIENF LCRAACVYYA TYTNNSEKGY AEWVINTRQV AQLRRKLELF TYLRFDLELT FVITSAQQPS TATSVDAPVQ THQIMYVPP GGPVPTKVTD YAWQTSTNPS VFWTEGNAPP RMSIPFISIG NAYSCFYDGW TQFSRNGVYG INTLNNMGTL Y MRHVNEAG QGPIKSTVRI YFKPKHVKAW VPRPPRLCQY EKQKNVNFTP TGVTTTRVGI TT UniProtKB: Genome polyprotein |
-Macromolecule #2: Capsid protein VP2
| Macromolecule | Name: Capsid protein VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Coxsackievirus B1 Coxsackievirus B1 |
| Molecular weight | Theoretical: 27.725402 KDa |
| Sequence | String: RVRSITLGNS TITTQECANV VVGYGVWPEY LKDNEATAED QPTQPDVATC RFYTLESVQW MKNSAGWWWK LPDALSQMGL FGQNMQYHY LGRTGYTIHV QCNASKFHQG CLLVVCVPEA EMGCSNLNNT PEFAELSGGD TARMFTDTQI GETNSKKVQT A VWNAGMGV ...String: RVRSITLGNS TITTQECANV VVGYGVWPEY LKDNEATAED QPTQPDVATC RFYTLESVQW MKNSAGWWWK LPDALSQMGL FGQNMQYHY LGRTGYTIHV QCNASKFHQG CLLVVCVPEA EMGCSNLNNT PEFAELSGGD TARMFTDTQI GETNSKKVQT A VWNAGMGV GVGNLTIYPH QWINLRTNNS ATIVMPYINS VPMDNMFRHN NLTLMIIPFV PLNYSEGSSP YVPITVTIAP MC AEYNGLR LA UniProtKB: Genome polyprotein |
-Macromolecule #3: Capsid protein VP3
| Macromolecule | Name: Capsid protein VP3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Coxsackievirus B1 Coxsackievirus B1 |
| Molecular weight | Theoretical: 25.485955 KDa |
| Sequence | String: GLPVMTTPGS TQFLTSDDFQ SPSAMPQFDV TPEMQIPGRV NNLMEIAEVD SVVPVNNTEA NVNSLKAYQI PVQSNSDNGK QVFGFPLQP GANGVLNRTL LGEILNYYTH WSGSIKLTFM FCGSAMATGK FLLAYSPPGA GVPKNRKDAM LGTHVIWDVG L QSSCVLCV ...String: GLPVMTTPGS TQFLTSDDFQ SPSAMPQFDV TPEMQIPGRV NNLMEIAEVD SVVPVNNTEA NVNSLKAYQI PVQSNSDNGK QVFGFPLQP GANGVLNRTL LGEILNYYTH WSGSIKLTFM FCGSAMATGK FLLAYSPPGA GVPKNRKDAM LGTHVIWDVG L QSSCVLCV PWISQTHYRY VVEDEYTAAG YITCWYQTNI VVPADVQSSC DILCFVSACN DFSVRMLKDT PFIR UniProtKB: Genome polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 10 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 295 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 0.1 µm / Nominal magnification: 150000 |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)