[English] 日本語
 Yorodumi
Yorodumi- EMDB-50392: Structure of a eukaryotic replisome stalled at a lagging-strand T... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of a eukaryotic replisome stalled at a lagging-strand Topoisomerase 1 cleavage complex with Tof1-Csm3 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA / polymerase / epsilon / PCNA / leading strand / human / replication / replisome / proofrerading | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 6.2 Å | |||||||||
 Authors Authors | Westhorpe R / Roske JJ / Yeeles JTP | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: Mechanisms controlling replication fork stalling and collapse at topoisomerase 1 cleavage complexes. Authors: Rose Westhorpe / Johann J Roske / Joseph T P Yeeles /  Abstract: Topoisomerase 1 cleavage complexes (Top1-ccs) comprise a DNA-protein crosslink and a single-stranded DNA break that can significantly impact the DNA replication machinery (replisome). Consequently, ...Topoisomerase 1 cleavage complexes (Top1-ccs) comprise a DNA-protein crosslink and a single-stranded DNA break that can significantly impact the DNA replication machinery (replisome). Consequently, inhibitors that trap Top1-ccs are used extensively in research and clinical settings to generate DNA replication stress, yet how the replisome responds upon collision with a Top1-cc remains obscure. By reconstituting collisions between budding yeast replisomes, assembled from purified proteins, and site-specific Top1-ccs, we have uncovered mechanisms underlying replication fork stalling and collapse. We find that stalled replication forks are surprisingly stable and that their stability is influenced by the template strand that Top1 is crosslinked to, the fork protection complex proteins Tof1-Csm3 (human TIMELESS-TIPIN), and the convergence of replication forks. Moreover, nascent-strand mapping and cryoelectron microscopy (cryo-EM) of stalled forks establishes replisome remodeling as a key factor in the initial response to Top1-ccs. These findings have important implications for the use of Top1 inhibitors in research and in the clinic. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_50392.map.gz emd_50392.map.gz | 2.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-50392-v30.xml emd-50392-v30.xml emd-50392.xml emd-50392.xml | 13.6 KB 13.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_50392_fsc.xml emd_50392_fsc.xml | 10.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_50392.png emd_50392.png | 92.5 KB | ||
| Filedesc metadata |  emd-50392.cif.gz emd-50392.cif.gz | 3.9 KB | ||
| Others |  emd_50392_additional_1.map.gz emd_50392_additional_1.map.gz emd_50392_half_map_1.map.gz emd_50392_half_map_1.map.gz emd_50392_half_map_2.map.gz emd_50392_half_map_2.map.gz | 86 MB 84.5 MB 84.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-50392 http://ftp.pdbj.org/pub/emdb/structures/EMD-50392 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50392 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50392 | HTTPS FTP |
-Validation report
| Summary document |  emd_50392_validation.pdf.gz emd_50392_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_50392_full_validation.pdf.gz emd_50392_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_50392_validation.xml.gz emd_50392_validation.xml.gz | 18 KB | Display | |
| Data in CIF |  emd_50392_validation.cif.gz emd_50392_validation.cif.gz | 23.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50392 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50392 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50392 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50392 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_50392.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_50392.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.86 Å | ||||||||||||||||||||||||||||||||||||
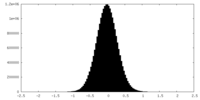
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Refined consensus, globally sharpened
| File | emd_50392_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refined consensus, globally sharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
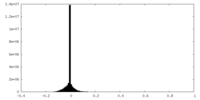
| Density Histograms |
-Half map: #1
| File | emd_50392_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_50392_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Budding yeast replisome stalled at a leading-strand Topoisomerase...
| Entire | Name: Budding yeast replisome stalled at a leading-strand Topoisomerase 1 cleavage complex. |
|---|---|
| Components |
|
-Supramolecule #1: Budding yeast replisome stalled at a leading-strand Topoisomerase...
| Supramolecule | Name: Budding yeast replisome stalled at a leading-strand Topoisomerase 1 cleavage complex. type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 39.9 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)