+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | ss-dsDNA-FANCD2-FANCI complex | |||||||||
 Map data Map data | Consensus map of ss-dsDNA-D2-I complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ss-dsDNA-FANCD2-FANCI / Fanconi Anemia / D2-I complex / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.59 Å | |||||||||
 Authors Authors | Alcon P / Passmore LA | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: FANCD2-FANCI surveys DNA and recognizes double- to single-stranded junctions. Authors: Pablo Alcón / Artur P Kaczmarczyk / Korak Kumar Ray / Themistoklis Liolios / Guillaume Guilbaud / Tamara Sijacki / Yichao Shen / Stephen H McLaughlin / Julian E Sale / Puck Knipscheer / ...Authors: Pablo Alcón / Artur P Kaczmarczyk / Korak Kumar Ray / Themistoklis Liolios / Guillaume Guilbaud / Tamara Sijacki / Yichao Shen / Stephen H McLaughlin / Julian E Sale / Puck Knipscheer / David S Rueda / Lori A Passmore /   Abstract: DNA crosslinks block DNA replication and are repaired by the Fanconi anaemia pathway. The FANCD2-FANCI (D2-I) protein complex is central to this process as it initiates repair by coordinating DNA ...DNA crosslinks block DNA replication and are repaired by the Fanconi anaemia pathway. The FANCD2-FANCI (D2-I) protein complex is central to this process as it initiates repair by coordinating DNA incisions around the lesion. However, D2-I is also known to have a more general role in DNA repair and in protecting stalled replication forks from unscheduled degradation. At present, it is unclear how DNA crosslinks are recognized and how D2-I functions in replication fork protection. Here, using single-molecule imaging, we show that D2-I is a sliding clamp that binds to and diffuses on double-stranded DNA. Notably, sliding D2-I stalls on encountering single-stranded-double-stranded (ss-ds) DNA junctions, structures that are generated when replication forks stall at DNA lesions. Using cryogenic electron microscopy, we determined structures of D2-I on DNA that show that stalled D2-I makes specific interactions with the ss-dsDNA junction that are distinct from those made by sliding D2-I. Thus, D2-I surveys dsDNA and, when it reaches an ssDNA gap, it specifically clamps onto ss-dsDNA junctions. Because ss-dsDNA junctions are found at stalled replication forks, D2-I can identify sites of DNA damage. Therefore, our data provide a unified molecular mechanism that reconciles the roles of D2-I in the recognition and protection of stalled replication forks in several DNA repair pathways. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_50353.map.gz emd_50353.map.gz | 129.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-50353-v30.xml emd-50353-v30.xml emd-50353.xml emd-50353.xml | 22.1 KB 22.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_50353.png emd_50353.png | 38.7 KB | ||
| Filedesc metadata |  emd-50353.cif.gz emd-50353.cif.gz | 7.5 KB | ||
| Others |  emd_50353_additional_1.map.gz emd_50353_additional_1.map.gz emd_50353_half_map_1.map.gz emd_50353_half_map_1.map.gz emd_50353_half_map_2.map.gz emd_50353_half_map_2.map.gz | 138.5 MB 129.2 MB 129.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-50353 http://ftp.pdbj.org/pub/emdb/structures/EMD-50353 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50353 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50353 | HTTPS FTP |
-Validation report
| Summary document |  emd_50353_validation.pdf.gz emd_50353_validation.pdf.gz | 886.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_50353_full_validation.pdf.gz emd_50353_full_validation.pdf.gz | 886.2 KB | Display | |
| Data in XML |  emd_50353_validation.xml.gz emd_50353_validation.xml.gz | 14.4 KB | Display | |
| Data in CIF |  emd_50353_validation.cif.gz emd_50353_validation.cif.gz | 17.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50353 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50353 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50353 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50353 | HTTPS FTP |
-Related structure data
| Related structure data |  9ffbM M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_50353.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_50353.map.gz / Format: CCP4 / Size: 163.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Consensus map of ss-dsDNA-D2-I complex | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.831 Å | ||||||||||||||||||||||||||||||||||||
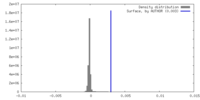
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Sharpened map of ss-dsDNA-D2-I complex
| File | emd_50353_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map of ss-dsDNA-D2-I complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
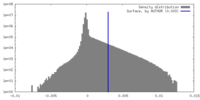
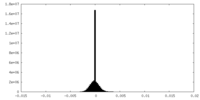
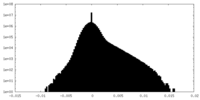
| Density Histograms |
-Half map: Half map 1 of ss-dsDNA-D2-I complex
| File | emd_50353_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 of ss-dsDNA-D2-I complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
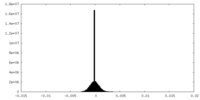
| Density Histograms |
-Half map: Half map 2 of ss-dsDNA-D2-I complex
| File | emd_50353_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 of ss-dsDNA-D2-I complex | ||||||||||||
| Projections & Slices |
| ||||||||||||
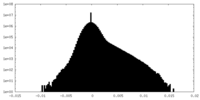
| Density Histograms |
- Sample components
Sample components
-Entire : ss-dsDNA-FANCD2-FANCI complex
| Entire | Name: ss-dsDNA-FANCD2-FANCI complex |
|---|---|
| Components |
|
-Supramolecule #1: ss-dsDNA-FANCD2-FANCI complex
| Supramolecule | Name: ss-dsDNA-FANCD2-FANCI complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 310 MDa |
-Macromolecule #1: DNA (5'-D(P*GP*CP*AP*GP*CP*TP*GP*TP*CP*TP*AP*GP*AP*GP*AP*CP*AP*TP...
| Macromolecule | Name: DNA (5'-D(P*GP*CP*AP*GP*CP*TP*GP*TP*CP*TP*AP*GP*AP*GP*AP*CP*AP*TP*CP*GP*AP*T)-3') type: dna / ID: 1 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 6.776392 KDa |
| Sequence | String: (DG)(DC)(DA)(DG)(DC)(DT)(DG)(DT)(DC)(DT) (DA)(DG)(DA)(DG)(DA)(DC)(DA)(DT)(DC)(DG) (DA)(DT) |
-Macromolecule #2: DNA (5'-D(P*CP*GP*AP*TP*GP*TP*CP*TP*CP*TP*AP*GP*AP*CP*AP*GP*CP*TP...
| Macromolecule | Name: DNA (5'-D(P*CP*GP*AP*TP*GP*TP*CP*TP*CP*TP*AP*GP*AP*CP*AP*GP*CP*TP*GP*C)-3') type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 6.109954 KDa |
| Sequence | String: (DC)(DG)(DA)(DT)(DG)(DT)(DC)(DT)(DC)(DT) (DA)(DG)(DA)(DC)(DA)(DG)(DC)(DT)(DG)(DC) |
-Macromolecule #3: Fanconi anemia protein FANCD2
| Macromolecule | Name: Fanconi anemia protein FANCD2 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 164.731344 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MVSKRKLSKI DAAEESSKTD LQSRCPETKR SRISDKRAPS QGGLENEGVF EELLRTSGII LKVGEGQNEI AVDQTAFQKK LRVALEKHP SYPGVVNEFI SGLESHIKDR SQFKNCLLPC TPARTEGSRT LVHSYCESLI KLLLGIKILQ PAVVTLLLEK I PEFFFDVV ...String: MVSKRKLSKI DAAEESSKTD LQSRCPETKR SRISDKRAPS QGGLENEGVF EELLRTSGII LKVGEGQNEI AVDQTAFQKK LRVALEKHP SYPGVVNEFI SGLESHIKDR SQFKNCLLPC TPARTEGSRT LVHSYCESLI KLLLGIKILQ PAVVTLLLEK I PEFFFDVV GTFGTNFPRL IVNQFKWLDG LLDSQDLVKK LMQMLSVSPV PIQHDIITSL PEILEDSQQN EVARELSCLL KQ GRRLTVP ILDALSRLDL DAELLAKVRQ SAMTIVPSVK LEDLPVVIKF ILHNVKAADA VEVISDLRKS LDLSSCVLPL QLL GSQRKL KSQAQASSSM SQVTTSQNCV KLLFDVIKLA VRFQKDVSEA WIKAIENSTS VSDHKVLDLI VLLLIHSTNS KNRK QTEKV LRSKIRLGCM PEQLMQNAFQ NHSMVIKDFF PSILSLAQTF LHSAHPAVVS FGSCMYKQAF AVFDSYCQQE VVCAL VTHV CSGNETELDI SLDVLTDLVI LHPSLLLRYA TFVKTILDSM QKLNPCQIRK LFYILSTLAF SQRQEGSYIQ DDMHMV IRK WLSSSVPNHK QMGIIGAVTM MGSVALKRNE ADGGLLERPE LSIECDGQLS TLLDLVGFCC EQTPEVLALY YDELANL IE KQKGNLDLQL LDKFGKSLVE DFPNDFVVDL SPTVDGSFLF PVKSLYNLDE DETQGAIAIN LLPLVSQSEP GRVADEMS N SRKRVVSPIC LSPCFRLLRL YTGEQNNGSL EEIDALLGCP LYLTDLEVEG KLDSLSKQER EFLCSLLFYA LNWFREVVN AFCQQQDAEM KGKVLTRLQN ITELQNVLGK CLAATPGYVP PPATFDSEAP EGVPSINAGG PVRKKNGKKR KSDSSKACSA ERTQADESS DGNQPDTELS ELEKSAAEKE TGNPLAQLQS YRPYFRELDL EVFSVLHCGL LTKSILDTEM HTEASEVVQL G PAELCFLL DDMCWKLEHV LTPGSTRRVP FLKERGNKDV GFSHLCQRSP KEVAVCVVKL LKPLCNHMEN MHNYFQTVIP NQ GVVDESG LNIQEYQLMS SCYHQLLLAF RLLFAWSGFS QHENSNLLRS ALQVLADRLK PGETEFLPLE ELISESFQYL LNF QASIPS FQCAFILTQV LMAISEKPMT GWKREKMASL AKQFLCQSWM KPGGDREKGS HFNSALHTLL CVYLEHTDNI LKAI EEISS VGVPELINSA KDGCSSTYPT LSRQTFPVFF RVMMAQLESS VKSIPAGKPS DSGEVQLEKL LKWNIAVRNF HILIN LVKV FDSRPVLSIC LKYGRLFVEA FLKLAMPLLD HSFKKHRDDV QSLLKTLQLS TRQLHHMCGH SKIHQDLGLT NHVPLL KKS LEQFVYRVKA MLAFNHCQEA FWVGVLKNRD LQGEEILSQA SAAPEEDSAE GSEEDTEDSA AEEPDGTDSD SGGAGRL EV LFQGPWSHPQ FEKGSAGSAA GSGAGWSHPQ FEK UniProtKB: Fanconi anemia group D2 protein |
-Macromolecule #4: Fanconi anemia complementation group I
| Macromolecule | Name: Fanconi anemia complementation group I / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 149.458297 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAQRILQLAA EGSPERLQEA LQGLTEGELG DMVTRQALRG RETAALLKGI FKGSPCSQQS GVLRRLQVYK HCVSLVESGD LHVGKVSEI IGLLMLEARQ LPGHALAELA TLFVEVIKRG SLSNGKSLEL FSTVLTALSN SKESLAYGKG ELNGEEFKKQ L INTLCSSK ...String: MAQRILQLAA EGSPERLQEA LQGLTEGELG DMVTRQALRG RETAALLKGI FKGSPCSQQS GVLRRLQVYK HCVSLVESGD LHVGKVSEI IGLLMLEARQ LPGHALAELA TLFVEVIKRG SLSNGKSLEL FSTVLTALSN SKESLAYGKG ELNGEEFKKQ L INTLCSSK WDPQCVIHLA NMFRDIPLSG EELQFVVEKV LRMFSKLDLQ EIPPLVYQLL LLSAKGSKKT VLEGIISFFN QL DKRQKEE QRVPQSADLE VATVPLDQLR HVEGTVILHI VSAINLDQDI GEELIKHLKT EQQKDPGKAL CPFSVSLLLS TAV KHRLQE QIFDFLKTSI TRSCKDLQIL QASKFLQDLC PQQYDVTAVI LEVVKNSAFG WDHVTQGLVD LGFSLMESYE PKKS FGGKA AETNLGLSKM PAQQACKLGA SILLETFKVH EPIRSDILEQ VLNRVLTKAA SPVSHFIDLL SNIVVSAPLV LQNSS SRVT ETFDNLSFLP IDTVQGLLRA VQPLLKVSMS VRDSLILVLQ KAIFSRQLDA RKAAVAGFLL LLRNFKILGS LTSSQC SQA IGATQVQADV HACYNSAANE AFCLEILGSL RRCLSQQADV RLMLYEGFYD VLRRNSQLAS SIMETLLSQI KQYYLPQ QD LLPPLKLEGC IMAQGDQIFL QEPLAHLLCC IQHCLAWYKS TVHLCKGAED EEEEEDVGFE QNFEEMLESV TRRMIKSE L EDFELDKSAD FSPSSGVGVK NNIYAIQVMG ICEVLIEYNF KIGNFSKNKF EDVLGLFTCY NKLSEILKEK AGKNKSTLG NRIARSFLSM GFVSTLLTAL FRDNAQSHEE SLAVLRSSTE FMRYAVSVAL QKVQQLEEMG QTDGPDGQNP EKMFQNLCKI TRVLLWRYT SIPTAVEESG KKKGKSISLL CLEGLLRIFN TMQQLYAARI PQFLQALDIT DGDAEEADIN VTEKAAFQIR Q FQRSLVNQ LSSAEDDFNS KETQLLITIL STLSKLLDPG SQQFLQFLTW TVKICKENAL EDLSCCKGLL TLLFSLHVLY KS PVSLLRE LAQDIHACLG DIDQDVEIES RSHFAIVNVK TAAPTVCLLV LGQADKVLEE VDWLIKRLTI LGSDTSEDST QAS NQTQAL EKGVILQLGT LLTVFHELVQ TALPAGSCVD SLLRSLSKTY AILTSLIKHY IQACRSTSNT VPGRLEKLVK LSGS HLTPQ CYSFITYVQN IHSESLSFAE EKKKKKKEDE TAVVSTVMAK VLRDTKPIPN LIFAIEQYEK FLIHLSKKSK VNLMQ YMKL STSRDFRINA SMLDSVLQEQ NTEDAENEPD NNQSGTAEQP DENQEPQKKR RRKK UniProtKB: Fanconi anemia group I protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER / Details: Ab initial model generated in RELION 4.0 |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.59 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 319378 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)