[English] 日本語
 Yorodumi
Yorodumi- EMDB-50062: The structure of nonameric pore of RN1 variant of actinoporin Fav -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | The structure of nonameric pore of RN1 variant of actinoporin Fav | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Actinoporin / Pore-forming toxin / Pore / nonamer / Transmembrane pore / TOXIN / Protein nanopore | ||||||||||||
| Biological species |  Orbicella faveolata (invertebrata) Orbicella faveolata (invertebrata) | ||||||||||||
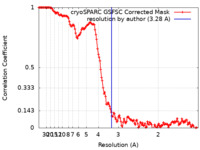
| Method | single particle reconstruction / cryo EM / Resolution: 3.28 Å | ||||||||||||
 Authors Authors | Solinc G / Srnko M / Anderluh G / Crnkovic A / Podobnik M | ||||||||||||
| Funding support |  Slovenia, 3 items Slovenia, 3 items
| ||||||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: The structure of nonameric pore of RN1 variant of actinoporin Fav Authors: Solinc G / Srnko M / Anderluh G / Crnkovic A / Podobnik M | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_50062.map.gz emd_50062.map.gz | 229.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-50062-v30.xml emd-50062-v30.xml emd-50062.xml emd-50062.xml | 19.8 KB 19.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_50062_fsc.xml emd_50062_fsc.xml | 13.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_50062.png emd_50062.png | 59.4 KB | ||
| Filedesc metadata |  emd-50062.cif.gz emd-50062.cif.gz | 6.8 KB | ||
| Others |  emd_50062_half_map_1.map.gz emd_50062_half_map_1.map.gz emd_50062_half_map_2.map.gz emd_50062_half_map_2.map.gz | 225.9 MB 225.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-50062 http://ftp.pdbj.org/pub/emdb/structures/EMD-50062 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50062 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50062 | HTTPS FTP |
-Validation report
| Summary document |  emd_50062_validation.pdf.gz emd_50062_validation.pdf.gz | 827.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_50062_full_validation.pdf.gz emd_50062_full_validation.pdf.gz | 827.2 KB | Display | |
| Data in XML |  emd_50062_validation.xml.gz emd_50062_validation.xml.gz | 22.1 KB | Display | |
| Data in CIF |  emd_50062_validation.cif.gz emd_50062_validation.cif.gz | 28.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50062 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50062 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50062 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50062 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_50062.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_50062.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.745 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map B
| File | emd_50062_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_50062_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Nonameric RN1-Fav pore
| Entire | Name: Nonameric RN1-Fav pore |
|---|---|
| Components |
|
-Supramolecule #1: Nonameric RN1-Fav pore
| Supramolecule | Name: Nonameric RN1-Fav pore / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Nonameric Fav pore prepared on 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC):sphingomyelin (1:1 molar ratio) membranes |
|---|---|
| Source (natural) | Organism:  Orbicella faveolata (invertebrata) Orbicella faveolata (invertebrata) |
-Macromolecule #1: Actinoporin
| Macromolecule | Name: Actinoporin / type: protein_or_peptide / ID: 1 Details: This protein was expressed with an N-terminal deletion of 67 residues compared to the wild type. The deletion construct has three additional residues at the N-terminal (S) from the expression system. Number of copies: 9 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Orbicella faveolata (invertebrata) Orbicella faveolata (invertebrata) |
| Molecular weight | Theoretical: 21.307543 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SELDSENDAA DIAAGTIIAG AELTFGLLQN LLYFFANVNR KCAVGVDNES GFRWQEGSTY FFSGTADENL PYSVSDGYAV LYGPRKTNG PVATGVVGVL AYYIPSIGKT LAVMWSVPFD YNFYQNWWNA KLYSGNQRAD YDHYVDLYYN ANPFKANGWH E RSLGSGLK ...String: SELDSENDAA DIAAGTIIAG AELTFGLLQN LLYFFANVNR KCAVGVDNES GFRWQEGSTY FFSGTADENL PYSVSDGYAV LYGPRKTNG PVATGVVGVL AYYIPSIGKT LAVMWSVPFD YNFYQNWWNA KLYSGNQRAD YDHYVDLYYN ANPFKANGWH E RSLGSGLK FCGSMSSSGQ ATLEIHVLKE SETCM |
-Macromolecule #2: Sphingomyelin C18
| Macromolecule | Name: Sphingomyelin C18 / type: ligand / ID: 2 / Number of copies: 45 / Formula: A1H8M |
|---|---|
| Molecular weight | Theoretical: 732.089 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: 150 mM NaCl, 50 mM Tris/HCl, 0.02 % Brij 35, pH 8 | ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.01 kPa | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number real images: 5147 / Average electron dose: 32.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 0.4 µm / Nominal magnification: 150000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: Other / Chain - Initial model type: experimental model Details: Pore structure of the same protein from related entries |
|---|---|
| Refinement | Space: REAL |
| Output model |  PDB-9eyq: |
 Movie
Movie Controller
Controller



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)