[English] 日本語
 Yorodumi
Yorodumi- EMDB-4892: Cryo-EM structure of E. coli RNA polymerase backtracked elongatio... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4892 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of E. coli RNA polymerase backtracked elongation complex bound to GreB transcription factor | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | E. coli RNA polymerase / backtracking / GreB / Elongation complex / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology informationDNA-templated transcription elongation / transcription elongation factor activity / RNA polymerase binding / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / regulation of DNA-templated transcription initiation / bacterial-type flagellum assembly / bacterial-type RNA polymerase core enzyme binding / cytosolic DNA-directed RNA polymerase complex ...DNA-templated transcription elongation / transcription elongation factor activity / RNA polymerase binding / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / regulation of DNA-templated transcription initiation / bacterial-type flagellum assembly / bacterial-type RNA polymerase core enzyme binding / cytosolic DNA-directed RNA polymerase complex / bacterial-type flagellum-dependent cell motility / translation elongation factor activity / nitrate assimilation / regulation of DNA-templated transcription elongation / transcription elongation factor complex / transcription antitermination / DNA-templated transcription initiation / cell motility / ribonucleoside binding / DNA-directed RNA polymerase / DNA-directed RNA polymerase activity / response to heat / protein-containing complex assembly / intracellular iron ion homeostasis / protein dimerization activity / response to antibiotic / magnesium ion binding / DNA binding / zinc ion binding / membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |     | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Abdelkareem M / Saint-Andre C | |||||||||
| Funding support |  France, 1 items France, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2019 Journal: Mol Cell / Year: 2019Title: Structural Basis of Transcription: RNA Polymerase Backtracking and Its Reactivation. Authors: Mo'men Abdelkareem / Charlotte Saint-André / Maria Takacs / Gabor Papai / Corinne Crucifix / Xieyang Guo / Julio Ortiz / Albert Weixlbaumer /  Abstract: Regulatory sequences or erroneous incorporations during DNA transcription cause RNA polymerase backtracking and inactivation in all kingdoms of life. Reactivation requires RNA transcript cleavage. ...Regulatory sequences or erroneous incorporations during DNA transcription cause RNA polymerase backtracking and inactivation in all kingdoms of life. Reactivation requires RNA transcript cleavage. Essential transcription factors (GreA and GreB, or TFIIS) accelerate this reaction. We report four cryo-EM reconstructions of Escherichia coli RNA polymerase representing the entire reaction pathway: (1) a backtracked complex; a backtracked complex with GreB (2) before and (3) after RNA cleavage; and (4) a reactivated, substrate-bound complex with GreB before RNA extension. Compared with eukaryotes, the backtracked RNA adopts a different conformation. RNA polymerase conformational changes cause distinct GreB states: a fully engaged GreB before cleavage; a disengaged GreB after cleavage; and a dislodged, loosely bound GreB removed from the active site to allow RNA extension. These reconstructions provide insight into the catalytic mechanism and dynamics of RNA cleavage and extension and suggest how GreB targets backtracked complexes without interfering with canonical transcription. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4892.map.gz emd_4892.map.gz | 78.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4892-v30.xml emd-4892-v30.xml emd-4892.xml emd-4892.xml | 24.8 KB 24.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4892.png emd_4892.png | 43.8 KB | ||
| Filedesc metadata |  emd-4892.cif.gz emd-4892.cif.gz | 9.7 KB | ||
| Others |  emd_4892_additional.map.gz emd_4892_additional.map.gz | 2.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4892 http://ftp.pdbj.org/pub/emdb/structures/EMD-4892 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4892 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4892 | HTTPS FTP |
-Validation report
| Summary document |  emd_4892_validation.pdf.gz emd_4892_validation.pdf.gz | 279 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_4892_full_validation.pdf.gz emd_4892_full_validation.pdf.gz | 278.1 KB | Display | |
| Data in XML |  emd_4892_validation.xml.gz emd_4892_validation.xml.gz | 6.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4892 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4892 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4892 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4892 | HTTPS FTP |
-Related structure data
| Related structure data |  6rinMC  4882C  4885C  4886C  4893C  6rh3C  6ri7C  6ri9C  6ripC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4892.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4892.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
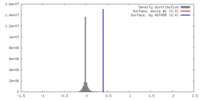
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: #1
| File | emd_4892_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
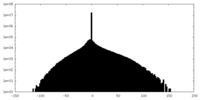
| Density Histograms |
- Sample components
Sample components
+Entire : Cryo-EM structure of E. coli RNA polymerase backtracked elongatio...
+Supramolecule #1: Cryo-EM structure of E. coli RNA polymerase backtracked elongatio...
+Supramolecule #2: RNA polymerase and GreB
+Supramolecule #3: Nucleic acids
+Macromolecule #1: Non-template DNA
+Macromolecule #2: Template DNA
+Macromolecule #3: Transcription elongation factor GreB
+Macromolecule #4: DNA-directed RNA polymerase subunit alpha
+Macromolecule #5: DNA-directed RNA polymerase subunit beta
+Macromolecule #6: DNA-directed RNA polymerase subunit beta'
+Macromolecule #7: DNA-directed RNA polymerase subunit omega
+Macromolecule #8: RNA
+Macromolecule #9: ZINC ION
+Macromolecule #10: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
| Details | Cryo-EM structure of E. coli RNA polymerase backtracked elongation complex bound to GreB transcription factor |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 51.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE / Details: Ab-initio reconstruction |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.7 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 574584 |
| Initial angle assignment | Type: NOT APPLICABLE / Software - Name: cryoSPARC |
| Final angle assignment | Type: NOT APPLICABLE / Software - Name: cryoSPARC |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)