[English] 日本語
 Yorodumi
Yorodumi- EMDB-48186: Antibody fragments from mAb21 and mAb824 bound to the adhesin pro... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Antibody fragments from mAb21 and mAb824 bound to the adhesin protein FimH containing alpha-methyl mannose | |||||||||||||||
 Map data Map data | Antibody fragments from mAb21 and mAb824 bound to the E. coli adhesin protein FimH containing alpha-methyl mannose | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Fimbrial tip / Lectin domain / Antibody fragments / Antibody-target complex / CELL ADHESION / CELL ADHESION-IMMUNE SYSTEM complex | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpilus tip / mechanosensory behavior / cell adhesion involved in single-species biofilm formation / pilus / cell-substrate adhesion / D-mannose binding / host cell membrane / cell adhesion Similarity search - Function | |||||||||||||||
| Biological species |   | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||||||||
 Authors Authors | Hvorecny KL / Magala P / Klevit RE / Kollman JM | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Antibodies disrupt bacterial adhesion by ligand mimicry and allosteric interference. Authors: Kelli L Hvorecny / Gianluca Interlandi / Tim S Veth / Pavel Aprikian / Anna Manchenko / Veronika L Tchesnokova / Miles S Dickinson / Joel D Quispe / Nicholas M Riley / Rachel E Klevit / ...Authors: Kelli L Hvorecny / Gianluca Interlandi / Tim S Veth / Pavel Aprikian / Anna Manchenko / Veronika L Tchesnokova / Miles S Dickinson / Joel D Quispe / Nicholas M Riley / Rachel E Klevit / Pearl Magala / Evgeni V Sokurenko / Justin M Kollman /  Abstract: A critical step in infections is the attachment of microorganisms to host cells using lectins that bind glycans, making lectins promising antimicrobial targets. Upon binding mannosylated glycans, ...A critical step in infections is the attachment of microorganisms to host cells using lectins that bind glycans, making lectins promising antimicrobial targets. Upon binding mannosylated glycans, FimH, an adhesin in E. coli, undergoes an allosteric transition from an inactive to an active conformation that can act as a catch-bond. Distinct monoclonal antibodies that alter FimH glycan binding are available, but the mechanisms of action remain unclear. Here, we use cryo-electron microscopy, mass spectrometry, adhesion assays, and molecular dynamics simulations to determine the structure-function relationships underlying antibody-FimH binding. Our study demonstrates four mechanisms of action: ligand mimicry by an N-linked, high-mannose glycan; stabilization of the ligand pocket in the inactive state; conformational trapping of the active and inactive states; and locking of the ligand pocket through long-range allosteric effects. These structures reveal multiple mechanisms of antibody responses to an allosteric protein and provide blueprints for antimicrobials that target adhesins. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_48186.map.gz emd_48186.map.gz | 12.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-48186-v30.xml emd-48186-v30.xml emd-48186.xml emd-48186.xml | 31.7 KB 31.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_48186.png emd_48186.png | 49 KB | ||
| Filedesc metadata |  emd-48186.cif.gz emd-48186.cif.gz | 7.6 KB | ||
| Others |  emd_48186_additional_1.map.gz emd_48186_additional_1.map.gz emd_48186_additional_2.map.gz emd_48186_additional_2.map.gz emd_48186_additional_3.map.gz emd_48186_additional_3.map.gz emd_48186_half_map_1.map.gz emd_48186_half_map_1.map.gz emd_48186_half_map_2.map.gz emd_48186_half_map_2.map.gz | 3.3 MB 391.5 MB 391.6 MB 392 MB 392 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-48186 http://ftp.pdbj.org/pub/emdb/structures/EMD-48186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-48186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-48186 | HTTPS FTP |
-Related structure data
| Related structure data |  9me7MC  9me4C  9me5C  9me6C  9ptmC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_48186.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_48186.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Antibody fragments from mAb21 and mAb824 bound to the E. coli adhesin protein FimH containing alpha-methyl mannose | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.124 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Antibody fragments from mAb21 and mAb824 bound to...
| File | emd_48186_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Antibody fragments from mAb21 and mAb824 bound to the E. coli adhesin protein FimH containing alpha-methyl mannose, masked around variable regions and FimH lectin domain | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Half maps of antibody fragments from mAb21 and...
| File | emd_48186_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half maps of antibody fragments from mAb21 and mAb824 bound to the E. coli adhesin protein FimH containing alpha-methyl mannose, masked around variable regions and FimH lectin domain | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Half maps of antibody fragments from mAb21 and...
| File | emd_48186_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half maps of antibody fragments from mAb21 and mAb824 bound to the E. coli adhesin protein FimH containing alpha-methyl mannose, masked around variable regions and FimH lectin domain | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of antibody fragments from mAb21 and...
| File | emd_48186_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of antibody fragments from mAb21 and mAb824 bound to the E. coli adhesin protein FimH containing alpha-methyl mannose | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of antibody fragments from mAb21 and...
| File | emd_48186_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of antibody fragments from mAb21 and mAb824 bound to the E. coli adhesin protein FimH containing alpha-methyl mannose | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Antibody fragments from mAb21 and mAb824 bound to the E. coli adh...
| Entire | Name: Antibody fragments from mAb21 and mAb824 bound to the E. coli adhesin protein FimH containing alpha-methyl mannose |
|---|---|
| Components |
|
-Supramolecule #1: Antibody fragments from mAb21 and mAb824 bound to the E. coli adh...
| Supramolecule | Name: Antibody fragments from mAb21 and mAb824 bound to the E. coli adhesin protein FimH containing alpha-methyl mannose type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|
-Supramolecule #2: Fimbrial tip
| Supramolecule | Name: Fimbrial tip / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: Antibody Fragments
| Supramolecule | Name: Antibody Fragments / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#5 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Type 1 fimbrin D-mannose specific adhesin
| Macromolecule | Name: Type 1 fimbrin D-mannose specific adhesin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 31.48826 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKRVITLFAV LLMGWSVNAW SFACKTANGT AIPIGGGSAN VYVNLAPVVN VGQNLVVDLS TQIFCHNDYP ETITDYVTLQ RGSAYGGVL SNFSGTVKYS GSSYPFPTTS ETPRVVYNSR TDKPWPVALY LTPVSSAGGV AIKAGSLIAV LILRQTNNYN S DDFQFVWN ...String: MKRVITLFAV LLMGWSVNAW SFACKTANGT AIPIGGGSAN VYVNLAPVVN VGQNLVVDLS TQIFCHNDYP ETITDYVTLQ RGSAYGGVL SNFSGTVKYS GSSYPFPTTS ETPRVVYNSR TDKPWPVALY LTPVSSAGGV AIKAGSLIAV LILRQTNNYN S DDFQFVWN IYANNDVVVP TGGCDVSARD VTVTLPDYPG SVPIPLTVYC AKSQNLGYYL SGTTADAGNS IFTNTASFSP AQ GVGVQLT RNGTIIPANN TVSLGAVGTS AVSLGLTANY ARTGGQVTAG NVQSIIGVTF VYQ UniProtKB: Type 1 fimbrin D-mannose specific adhesin |
-Macromolecule #2: mAb824 Heavy Chain Fragment
| Macromolecule | Name: mAb824 Heavy Chain Fragment / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 22.526971 KDa |
| Sequence | String: EIQLQQSGPE RMKPGASVKI SCKASGYSFT TYYIHWVKQS HGRSLEWIGY IDPFNDDTNY NQKFKGKATL TVDKSSSTAY MHLSSLTSE DSAVYYCARS YYGSLDYWGQ GTTLTVSSAK TTPPSVYPLA PGSAAQTNSM VTLGCLVKGY FPEPVTVTWN S GSLSSGVH ...String: EIQLQQSGPE RMKPGASVKI SCKASGYSFT TYYIHWVKQS HGRSLEWIGY IDPFNDDTNY NQKFKGKATL TVDKSSSTAY MHLSSLTSE DSAVYYCARS YYGSLDYWGQ GTTLTVSSAK TTPPSVYPLA PGSAAQTNSM VTLGCLVKGY FPEPVTVTWN S GSLSSGVH TFPAVLQSDL YTLSSSVTVP SSTWPSETVT CNVAHPASST |
-Macromolecule #3: mAb21 Heavy Chain Fragment
| Macromolecule | Name: mAb21 Heavy Chain Fragment / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 21.143547 KDa |
| Sequence | String: EVLLKQSGPE KVKPGASVKI PCKASGYTFT DYNIDWVKQS HGTSLEWIGH LDPNSGGTVY NQKFRGKATL TVDKSSSTAY LELRSLTSE DTAVYYCARS TMGVYRSDGY YAMDYWGQGT SVTVSSAKTT PPSVYPLAPG CGDTTGSSVT LGCLVKGYFP E SVTVTWNS ...String: EVLLKQSGPE KVKPGASVKI PCKASGYTFT DYNIDWVKQS HGTSLEWIGH LDPNSGGTVY NQKFRGKATL TVDKSSSTAY LELRSLTSE DTAVYYCARS TMGVYRSDGY YAMDYWGQGT SVTVSSAKTT PPSVYPLAPG CGDTTGSSVT LGCLVKGYFP E SVTVTWNS GSLSSSVHTF PALLQSGLYT MSSSVTVPSS |
-Macromolecule #4: mAb824 Light Chain Fragment
| Macromolecule | Name: mAb824 Light Chain Fragment / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 21.757852 KDa |
| Sequence | String: DIQMTQTTSS LSASLGDRVT ISCRASQGVN NYLNWYQQKP DGSVKLLIYY TSNLHSGAPS RFSGSGSGTD YSLTISNLEQ EDIATYFCQ QANMVPWTFG GGTKLEIKRA DAAPTVSIFP PSSEQLTSGG ASVVCFLNNF YPKDINVKWK IDGSERQNGV L NSWTDQDS ...String: DIQMTQTTSS LSASLGDRVT ISCRASQGVN NYLNWYQQKP DGSVKLLIYY TSNLHSGAPS RFSGSGSGTD YSLTISNLEQ EDIATYFCQ QANMVPWTFG GGTKLEIKRA DAAPTVSIFP PSSEQLTSGG ASVVCFLNNF YPKDINVKWK IDGSERQNGV L NSWTDQDS KDSTYSMSST LTLTKDEYER HNSYTCEAR |
-Macromolecule #5: mAb21 Light Chain Fragment
| Macromolecule | Name: mAb21 Light Chain Fragment / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 19.737736 KDa |
| Sequence | String: QIVLTQSPAI MSASLGEEIT LTCSASSSIS YMHWYQQKSG TSPKILIYST SNQASGVPSR FSGSGSGTFY SLTISSVEAE DAADYYCHQ WSSYPWTFGG GTKLEIKRAD AAPTVSIFPP SSEQLTSGGA SVVCFLNNFY PKDINVKWKI DGSERQNGVL N SWTDQDSK DSTYSMSSTL TLTK |
-Macromolecule #6: methyl alpha-D-mannopyranoside
| Macromolecule | Name: methyl alpha-D-mannopyranoside / type: ligand / ID: 6 / Number of copies: 1 / Formula: MMA |
|---|---|
| Molecular weight | Theoretical: 194.182 Da |
| Chemical component information | 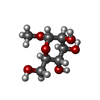 ChemComp-MMA: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 7832 / Average electron dose: 42.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.75 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT | ||||||
| Output model |  PDB-9me7: |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






























































