+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human muscle nAChR ACh-bound state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | human muscle / nicotinic acetylcholine receptor / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpostsynaptic membrane organization / skeletal muscle tissue growth / musculoskeletal movement / Highly sodium permeable postsynaptic acetylcholine nicotinic receptors / Highly calcium permeable nicotinic acetylcholine receptors / Highly calcium permeable postsynaptic nicotinic acetylcholine receptors / acetylcholine receptor activity / acetylcholine-gated channel complex / behavioral response to nicotine / neuromuscular synaptic transmission ...postsynaptic membrane organization / skeletal muscle tissue growth / musculoskeletal movement / Highly sodium permeable postsynaptic acetylcholine nicotinic receptors / Highly calcium permeable nicotinic acetylcholine receptors / Highly calcium permeable postsynaptic nicotinic acetylcholine receptors / acetylcholine receptor activity / acetylcholine-gated channel complex / behavioral response to nicotine / neuromuscular synaptic transmission / acetylcholine-gated monoatomic cation-selective channel activity / muscle cell development / acetylcholine binding / synaptic transmission, cholinergic / monoatomic cation transmembrane transporter activity / nervous system process / acetylcholine receptor signaling pathway / postsynaptic specialization membrane / neuromuscular process / muscle cell cellular homeostasis / neuromuscular junction development / ligand-gated monoatomic ion channel activity / membrane depolarization / monoatomic cation transport / neuronal action potential / skeletal muscle contraction / presynaptic modulation of chemical synaptic transmission / muscle contraction / response to nicotine / neuromuscular junction / regulation of membrane potential / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / neuron cellular homeostasis / transmembrane signaling receptor activity / presynapse / channel activity / monoatomic ion transmembrane transport / chemical synaptic transmission / postsynaptic membrane / neuron projection / synapse / cell surface / signal transduction / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.06 Å | |||||||||
 Authors Authors | Li H / Hibbs RE | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2025 Journal: Cell / Year: 2025Title: Autoimmune mechanisms elucidated through muscle acetylcholine receptor structures. Authors: Huanhuan Li / Minh C Pham / Jinfeng Teng / Kevin C O'Connor / Colleen M Noviello / Ryan E Hibbs /  Abstract: Skeletal muscle contraction is triggered by acetylcholine (ACh) binding to its ionotropic receptors (AChRs) at neuromuscular junctions. In myasthenia gravis (MG), autoantibodies target AChRs, ...Skeletal muscle contraction is triggered by acetylcholine (ACh) binding to its ionotropic receptors (AChRs) at neuromuscular junctions. In myasthenia gravis (MG), autoantibodies target AChRs, disrupting neurotransmission and causing muscle weakness. While treatments exist, variable patient responses suggest pathogenic heterogeneity. Progress in understanding the molecular basis of MG has been limited by the absence of structures of intact human muscle AChRs. Here, we present high-resolution cryoelectron microscopy (cryo-EM) structures of the human adult AChR in different functional states. Using six MG patient-derived monoclonal antibodies, we mapped distinct epitopes involved in diverse pathogenic mechanisms, including receptor blockade, internalization, and complement activation. Electrophysiological and binding assays revealed how these autoantibodies directly inhibit AChR channel activation. These findings provide critical insights into MG immunopathogenesis, uncovering unrecognized antibody epitope diversity and modes of receptor inhibition, and provide a framework for developing personalized therapies targeting antibody-mediated autoimmune disorders. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_47005.map.gz emd_47005.map.gz | 230.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-47005-v30.xml emd-47005-v30.xml emd-47005.xml emd-47005.xml | 23.1 KB 23.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_47005_fsc.xml emd_47005_fsc.xml | 13.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_47005.png emd_47005.png | 139 KB | ||
| Filedesc metadata |  emd-47005.cif.gz emd-47005.cif.gz | 7.4 KB | ||
| Others |  emd_47005_half_map_1.map.gz emd_47005_half_map_1.map.gz emd_47005_half_map_2.map.gz emd_47005_half_map_2.map.gz | 226.5 MB 226.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-47005 http://ftp.pdbj.org/pub/emdb/structures/EMD-47005 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-47005 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-47005 | HTTPS FTP |
-Related structure data
| Related structure data |  9dmhMC  9dmgC  9dmjC  9dmkC  9dmlC  9dmqC  9dmsC  9dmtC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_47005.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_47005.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.935 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_47005_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_47005_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human muscle nicotinic acetylcholine receptor
| Entire | Name: Human muscle nicotinic acetylcholine receptor |
|---|---|
| Components |
|
-Supramolecule #1: Human muscle nicotinic acetylcholine receptor
| Supramolecule | Name: Human muscle nicotinic acetylcholine receptor / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Acetylcholine receptor subunit alpha
| Macromolecule | Name: Acetylcholine receptor subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 51.889266 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEPWPLLLLF SLCSAGLVLG SEHETRLVAK LFKDYSSVVR PVEDHRQVVE VTVGLQLIQL INVDEVNQIV TTNVRLKQQW VDYNLKWNP DDYGGVKKIH IPSEKIWRPD LVLYNNADGD FAIVKFTKVL LQYTGHITWT PPAIFKSYCE IIVTHFPFDE Q NCSMKLGT ...String: MEPWPLLLLF SLCSAGLVLG SEHETRLVAK LFKDYSSVVR PVEDHRQVVE VTVGLQLIQL INVDEVNQIV TTNVRLKQQW VDYNLKWNP DDYGGVKKIH IPSEKIWRPD LVLYNNADGD FAIVKFTKVL LQYTGHITWT PPAIFKSYCE IIVTHFPFDE Q NCSMKLGT WTYDGSVVAI NPESDQPDLS NFMESGEWVI KESRGWKHSV TYSCCPDTPY LDITYHFVMQ RLPLYFIVNV II PCLLFSF LTGLVFYLPT DSGEKMTLSI SVLLSLTVFL LVIVELIPST SSAVPLIGKY MLFTMVFVIA SIIITVIVIN THH RSPSTH VMPNWVRKVF IDTIPNIMFF STMKRPSREK QDKKIFTEDI DISDISGKPG PPPMGFHSPL IKHPEVKSAI EGIK YIAET MKSDQESNNA AAEWKYVAMV MDHILLGVFM LVCIIGTLAV FAGRLIELNQ QG UniProtKB: Acetylcholine receptor subunit alpha |
-Macromolecule #2: Acetylcholine receptor subunit beta
| Macromolecule | Name: Acetylcholine receptor subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 57.00041 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MTPGALLMLL GALGAPLAPG VRGSEAEGRL REKLFSGYDS SVRPAREVGD RVRVSVGLIL AQLISLNEKD EEMSTKVYLD LEWTDYRLS WDPAEHDGID SLRITAESVW LPDVVLLNNN DGNFDVALDI SVVVSSDGSV RWQPPGIYRS SCSIQVTYFP F DWQNCTMV ...String: MTPGALLMLL GALGAPLAPG VRGSEAEGRL REKLFSGYDS SVRPAREVGD RVRVSVGLIL AQLISLNEKD EEMSTKVYLD LEWTDYRLS WDPAEHDGID SLRITAESVW LPDVVLLNNN DGNFDVALDI SVVVSSDGSV RWQPPGIYRS SCSIQVTYFP F DWQNCTMV FSSYSYDSSE VSLQTGLGPD GQGHQEIHIH EGTFIENGQW EIIHKPSRLI QPPGDPRGGR EGQRQEVIFY LI IRRKPLF YLVNVIAPCI LITLLAIFVF YLPPDAGEKM GLSIFALLTL TVFLLLLADK VPETSLSVPI IIKYLMFTMV LVT FSVILS VVVLNLHHRS PHTHQMPLWV RQIFIHKLPL YLRLKRPKPE RDLMPEPPHC SSPGSGWGRG TDEYFIRKPP SDFL FPKPN RFQPELSAPD LRRFIDGPNR AVALLPELRE VVSSISYIAR QLQEQEDHDA LKEDWQFVAM VVDRLFLWTF IIFTS VGTL VIFLDATYHL PPPDPFPSR UniProtKB: Acetylcholine receptor subunit beta |
-Macromolecule #3: Acetylcholine receptor subunit delta
| Macromolecule | Name: Acetylcholine receptor subunit delta / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 58.955805 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEGPVLTLGL LAALAVCGSW GLNEEERLIR HLFQEKGYNK ELRPVAHKEE SVDVALALTL SNLISLKEVE ETLTTNVWIE HGWTDNRLK WNAEEFGNIS VLRLPPDMVW LPEIVLENNN DGSFQISYSC NVLVYHYGFV YWLPPAIFRS SCPISVTYFP F DWQNCSLK ...String: MEGPVLTLGL LAALAVCGSW GLNEEERLIR HLFQEKGYNK ELRPVAHKEE SVDVALALTL SNLISLKEVE ETLTTNVWIE HGWTDNRLK WNAEEFGNIS VLRLPPDMVW LPEIVLENNN DGSFQISYSC NVLVYHYGFV YWLPPAIFRS SCPISVTYFP F DWQNCSLK FSSLKYTAKE ITLSLKQDAK ENRTYPVEWI IIDPEGFTEN GEWEIVHRPA RVNVDPRAPL DSPSRQDITF YL IIRRKPL FYIINILVPC VLISFMVNLV FYLPADSGEK TSVAISVLLA QSVFLLLISK RLPATSMAIP LIGKFLLFGM VLV TMVVVI CVIVLNIHFR TPSTHVLSEG VKKLFLETLP ELLHMSRPAE DGPSPGALVR RSSSLGYISK AEEYFLLKSR SDLM FEKQS ERHGLARRLT TARRPPASSE QAQQELFNEL KPAVDGANFI VNHMRDQNNY NEEKDSWNRV ARTVDRLCLF VVTPV MVVG TAWIFLQGVY NQPPPQPFPG DPYSYNVQDK RFI UniProtKB: Acetylcholine receptor subunit delta |
-Macromolecule #4: Acetylcholine receptor subunit epsilon
| Macromolecule | Name: Acetylcholine receptor subunit epsilon / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 54.743754 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MARAPLGVLL LLGLLGRGVG KNEELRLYHH LFNNYDPGSR PVREPEDTVT ISLKVTLTNL ISLNEKEETL TTSVWIGIDW QDYRLNYSK DDFGGIETLR VPSELVWLPE IVLENNIDGQ FGVAYDANVL VYEGGSVTWL PPAIYRSVCA VEVTYFPFDW Q NCSLIFRS ...String: MARAPLGVLL LLGLLGRGVG KNEELRLYHH LFNNYDPGSR PVREPEDTVT ISLKVTLTNL ISLNEKEETL TTSVWIGIDW QDYRLNYSK DDFGGIETLR VPSELVWLPE IVLENNIDGQ FGVAYDANVL VYEGGSVTWL PPAIYRSVCA VEVTYFPFDW Q NCSLIFRS QTYNAEEVEF TFAVDNDGKT INKIDIDTEA YTENGEWAID FCPGVIRRHH GGATDGPGET DVIYSLIIRR KP LFYVINI IVPCVLISGL VLLAYFLPAQ AGGQKCTVSI NVLLAQTVFL FLIAQKIPET SLSVPLLGRF LIFVMVVATL IVM NCVIVL NVSQRTPTTH AMSPRLRHVL LELLPRLLGS PPPPEAPRAA SPPRRASSVG LLLRAEELIL KKPRSELVFE GQRH RQGTW TAAFCQSLGA AAPEVRCCVD AVNFVAESTR DQEATGEEVS DWVRMGNALD NICFWAALVL FSVGSSLIFL GAYFN RVPD LPYAPCIQP UniProtKB: Acetylcholine receptor subunit epsilon |
-Macromolecule #10: ACETYLCHOLINE
| Macromolecule | Name: ACETYLCHOLINE / type: ligand / ID: 10 / Number of copies: 2 / Formula: ACH |
|---|---|
| Molecular weight | Theoretical: 146.207 Da |
| Chemical component information | 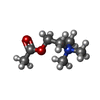 ChemComp-ACH: |
-Macromolecule #11: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 11 / Number of copies: 3 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-POV: |
-Macromolecule #12: water
| Macromolecule | Name: water / type: ligand / ID: 12 / Number of copies: 2 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.4000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





































