+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4668 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cas1-Cas2-Csn2-DNA complex from the Type II-A CRISPR-Cas system | |||||||||
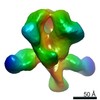
 Map data Map data | Cas1(8)-Cas2(4)-Csn2(8) DNA-bound monomer complex deposition map with C1 symmetry. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | CRISPR / Cas / DNA binding / Nuclease / Adaptation / Spacer acquisition / DNA BINDING PROTEIN / Protein complex / Protein-DNA complex / Calcium / Metal-binding / Antiviral | |||||||||
| Function / homology |  Function and homology information Function and homology informationmaintenance of CRISPR repeat elements / RNA endonuclease activity / DNA endonuclease activity / defense response to virus / Hydrolases; Acting on ester bonds / DNA binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Streptococcus thermophilus (bacteria) / Streptococcus thermophilus (bacteria) /  | |||||||||
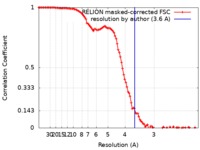
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Wilkinson M / Drabavicius G | |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2019 Journal: Mol Cell / Year: 2019Title: Structure of the DNA-Bound Spacer Capture Complex of a Type II CRISPR-Cas System. Authors: Martin Wilkinson / Gediminas Drabavicius / Arunas Silanskas / Giedrius Gasiunas / Virginijus Siksnys / Dale B Wigley /  Abstract: CRISPR and associated Cas proteins function as an adaptive immune system in prokaryotes to combat bacteriophage infection. During the immunization step, new spacers are acquired by the CRISPR ...CRISPR and associated Cas proteins function as an adaptive immune system in prokaryotes to combat bacteriophage infection. During the immunization step, new spacers are acquired by the CRISPR machinery, but the molecular mechanism of spacer capture remains enigmatic. We show that the Cas9, Cas1, Cas2, and Csn2 proteins of a Streptococcus thermophilus type II-A CRISPR-Cas system form a complex and provide cryoelectron microscopy (cryo-EM) structures of three different assemblies. The predominant form, with the stoichiometry Cas1-Cas2-Csn2, referred to as monomer, contains ∼30 bp duplex DNA bound along a central channel. A minor species, termed a dimer, comprises two monomers that sandwich a further eight Cas1 and four Cas2 subunits and contains two DNA ∼30-bp duplexes within the channel. A filamentous form also comprises Cas1-Cas2-Csn2 units (typically 2-6) but with a different Cas1-Cas2 interface between them and a continuous DNA duplex running along a central channel. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4668.map.gz emd_4668.map.gz | 89.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4668-v30.xml emd-4668-v30.xml emd-4668.xml emd-4668.xml | 24.2 KB 24.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4668_fsc.xml emd_4668_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_4668.png emd_4668.png | 101.5 KB | ||
| Filedesc metadata |  emd-4668.cif.gz emd-4668.cif.gz | 7.4 KB | ||
| Others |  emd_4668_additional.map.gz emd_4668_additional.map.gz | 89.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4668 http://ftp.pdbj.org/pub/emdb/structures/EMD-4668 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4668 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4668 | HTTPS FTP |
-Related structure data
| Related structure data |  6qxfMC  4670C  4671C  6qxtC  6qy3C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4668.map.gz / Format: CCP4 / Size: 98.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4668.map.gz / Format: CCP4 / Size: 98.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cas1(8)-Cas2(4)-Csn2(8) DNA-bound monomer complex deposition map with C1 symmetry. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.085 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
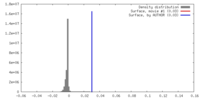
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Supplementary map refined with D2 symmetry to aid model building
| File | emd_4668_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Supplementary map refined with D2 symmetry to aid model building | ||||||||||||
| Projections & Slices |
| ||||||||||||
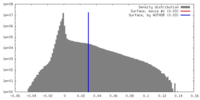
| Density Histograms |
- Sample components
Sample components
-Entire : Cas1-Cas2-Csn2-DNA monomer complex
| Entire | Name: Cas1-Cas2-Csn2-DNA monomer complex |
|---|---|
| Components |
|
-Supramolecule #1: Cas1-Cas2-Csn2-DNA monomer complex
| Supramolecule | Name: Cas1-Cas2-Csn2-DNA monomer complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Molecular weight | Theoretical: 530 KDa |
-Supramolecule #2: Cas1-Cas2-Csn2
| Supramolecule | Name: Cas1-Cas2-Csn2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Streptococcus thermophilus (bacteria) Streptococcus thermophilus (bacteria) |
-Supramolecule #3: DNA
| Supramolecule | Name: DNA / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #4-#5 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: CRISPR-associated protein Csn2
| Macromolecule | Name: CRISPR-associated protein Csn2 / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Streptococcus thermophilus (bacteria) Streptococcus thermophilus (bacteria) |
| Molecular weight | Theoretical: 25.533473 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKINFSLLDE PMEVNLGTVL VIEDVSVFAQ LVKEFYQYDE QSNLTIFDSK IRSIRSSELL LITDILGYDI NTSQVLKLLH TDIVSQLND KPEVRSEIDS LVSLITDIIM AECIENELDI EYDEITLLEL IKALGVRIET KSCTVFEKIF EILQIFKYLV K KRILVFVN ...String: MKINFSLLDE PMEVNLGTVL VIEDVSVFAQ LVKEFYQYDE QSNLTIFDSK IRSIRSSELL LITDILGYDI NTSQVLKLLH TDIVSQLND KPEVRSEIDS LVSLITDIIM AECIENELDI EYDEITLLEL IKALGVRIET KSCTVFEKIF EILQIFKYLV K KRILVFVN SLSYFSKDEI YQILEYTKLS QADVLFLEPR QIEGIQQFIL DKDYILMPYN N UniProtKB: CRISPR-associated protein Csn2 |
-Macromolecule #2: CRISPR-associated endonuclease Cas1
| Macromolecule | Name: CRISPR-associated endonuclease Cas1 / type: protein_or_peptide / ID: 2 / Number of copies: 8 / Enantiomer: LEVO / EC number: Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:  Streptococcus thermophilus (bacteria) Streptococcus thermophilus (bacteria) |
| Molecular weight | Theoretical: 35.363461 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAGWRTVVVN IHSKLSYKNN HLIFRNSYKT EMIHLSEIDI LLLETTDIVL TTMLVKRLVD ENILVIFCDD KRLPTAFLTP YYARHDSSL QIARQIAWKE NVKCEVWTAI IAQKILNQSY YLGECSFFEK SQSIMELYHG LERFDPSNRE GHSARIYFNT L FGNDFTRE ...String: MAGWRTVVVN IHSKLSYKNN HLIFRNSYKT EMIHLSEIDI LLLETTDIVL TTMLVKRLVD ENILVIFCDD KRLPTAFLTP YYARHDSSL QIARQIAWKE NVKCEVWTAI IAQKILNQSY YLGECSFFEK SQSIMELYHG LERFDPSNRE GHSARIYFNT L FGNDFTRE SDNDINAALD YGYTLLLSMF AREVVVCGCM TQIGLKHANQ FNQFNLASDI MEPFRPIIDR IVYQNRHNNF VK IKKELFS IFSETYLYNG KEMYLSNIVS DYTKKVIKAL NQLGEEIPEF RILESGWSHP QFEKA UniProtKB: CRISPR-associated endonuclease Cas1 |
-Macromolecule #3: CRISPR-associated endoribonuclease Cas2
| Macromolecule | Name: CRISPR-associated endoribonuclease Cas2 / type: protein_or_peptide / ID: 3 / Number of copies: 4 / Enantiomer: LEVO / EC number: Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:  Streptococcus thermophilus (bacteria) Streptococcus thermophilus (bacteria) |
| Molecular weight | Theoretical: 13.43156 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSYRYMRMIL MFDMPTDTAE ERKAYRKFRK FLLSEGFIMH QFSVYSKLLL NHTANTAMVG RLKANNPKKG NITILTVTEK QFARMIYLY GDKNTSIANS EERLVFLGDN YCDED UniProtKB: CRISPR-associated endoribonuclease Cas2 |
-Macromolecule #4: DNA (25-MER)
| Macromolecule | Name: DNA (25-MER) / type: dna / ID: 4 Details: Ordered portion representing the mixed DNA fragments bound to the complex after sonicating cells and treating with DNaseI. Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 7.559863 KDa |
| Sequence | String: (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DT)(DT)(DT)(DT)(DT) |
-Macromolecule #5: DNA (25-MER)
| Macromolecule | Name: DNA (25-MER) / type: dna / ID: 5 Details: Ordered portion representing the mixed DNA fragments bound to the complex after sonicating cells and treating with DNaseI. Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 7.785214 KDa |
| Sequence | String: (DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA) (DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA)(DA) (DA)(DA)(DA)(DA)(DA) |
-Macromolecule #6: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 6 / Number of copies: 8 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.05 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: Buffer solution was filtered | ||||||||||||
| Grid | Model: C-flat-2/1 / Material: COPPER / Mesh: 400 / Support film - Material: GRAPHENE OXIDE / Support film - topology: CONTINUOUS Details: Graphene oxide sheets were applied to the grid prior to sample application | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: 15 s wait time, 1 s blot time.. | ||||||||||||
| Details | Sample was DNaseI treated followed by gel filtration. Peak fractions were concentrated prior to making grids. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Number grids imaged: 1 / Number real images: 4908 / Average exposure time: 1.0 sec. / Average electron dose: 92.8 e/Å2 / Details: Images were collected in movie mode with 39 frames |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated magnification: 129032 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.3 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Details | Rounds of manual fitting in Coot, jelly-body refinement in REFMAC and real-space refinement in PHENIX | ||||||||||
| Refinement | Space: REAL / Protocol: OTHER Target criteria: Correlation coefficient and visual inspection | ||||||||||
| Output model |  PDB-6qxf: |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)