+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
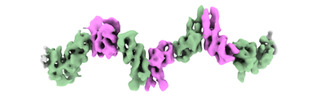
| タイトル | Cryo-EM structure of the ZBTB9 BTB domain filament | |||||||||
 マップデータ マップデータ | primary map | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | BTB domain / transcription factor / ZBTB protein / transcription | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報DNA-binding transcription factor activity, RNA polymerase II-specific / RNA polymerase II cis-regulatory region sequence-specific DNA binding / regulation of transcription by RNA polymerase II / zinc ion binding / identical protein binding / nucleus 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
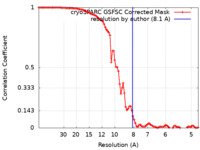
| 手法 | らせん対称体再構成法 / クライオ電子顕微鏡法 / 解像度: 8.1 Å | |||||||||
 データ登録者 データ登録者 | Park J / Hunkeler M / Fischer ES | |||||||||
| 資金援助 | 1件
| |||||||||
 引用 引用 |  ジャーナル: Mol Cell / 年: 2024 ジャーナル: Mol Cell / 年: 2024タイトル: Polymerization of ZBTB transcription factors regulates chromatin occupancy. 著者: Paul M C Park / Jiho Park / Jared Brown / Moritz Hunkeler / Shourya S Roy Burman / Katherine A Donovan / Hojong Yoon / Radosław P Nowak / Mikołaj Słabicki / Benjamin L Ebert / Eric S Fischer /  要旨: BCL6, an oncogenic transcription factor (TF), forms polymers in the presence of a small-molecule molecular glue that stabilizes a complementary interface between homodimers of BCL6's broad-complex, ...BCL6, an oncogenic transcription factor (TF), forms polymers in the presence of a small-molecule molecular glue that stabilizes a complementary interface between homodimers of BCL6's broad-complex, tramtrack, and bric-à-brac (BTB) domain. The BTB domains of other proteins, including a large class of TFs, have similar architectures and symmetries, raising the possibility that additional BTB proteins self-assemble into higher-order structures. Here, we surveyed 189 human BTB proteins with a cellular fluorescent reporter assay and identified 18 ZBTB TFs that show evidence of polymerization. Through biochemical and cryoelectron microscopy (cryo-EM) studies, we demonstrate that these ZBTB TFs polymerize into filaments. We found that BTB-domain-mediated polymerization of ZBTB TFs enhances chromatin occupancy within regions containing homotypic clusters of TF binding sites, leading to repression of target genes. Our results reveal a role of higher-order structures in regulating ZBTB TFs and suggest an underappreciated role for TF polymerization in modulating gene expression. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_44391.map.gz emd_44391.map.gz | 37.6 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-44391-v30.xml emd-44391-v30.xml emd-44391.xml emd-44391.xml | 20.4 KB 20.4 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_44391_fsc.xml emd_44391_fsc.xml | 9 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_44391.png emd_44391.png | 31.4 KB | ||
| マスクデータ |  emd_44391_msk_1.map emd_44391_msk_1.map | 75.1 MB |  マスクマップ マスクマップ | |
| Filedesc metadata |  emd-44391.cif.gz emd-44391.cif.gz | 6.4 KB | ||
| その他 |  emd_44391_additional_1.map.gz emd_44391_additional_1.map.gz emd_44391_half_map_1.map.gz emd_44391_half_map_1.map.gz emd_44391_half_map_2.map.gz emd_44391_half_map_2.map.gz | 70.9 MB 69.7 MB 69.7 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44391 http://ftp.pdbj.org/pub/emdb/structures/EMD-44391 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44391 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44391 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_44391_validation.pdf.gz emd_44391_validation.pdf.gz | 701.3 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_44391_full_validation.pdf.gz emd_44391_full_validation.pdf.gz | 700.9 KB | 表示 | |
| XML形式データ |  emd_44391_validation.xml.gz emd_44391_validation.xml.gz | 17 KB | 表示 | |
| CIF形式データ |  emd_44391_validation.cif.gz emd_44391_validation.cif.gz | 21.9 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44391 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44391 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44391 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44391 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  9b9vMC  9b9rC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_44391.map.gz / 形式: CCP4 / 大きさ: 75.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_44391.map.gz / 形式: CCP4 / 大きさ: 75.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | primary map | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 2.35 Å | ||||||||||||||||||||||||||||||||||||
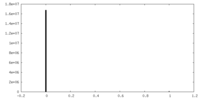
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-マスク #1
| ファイル |  emd_44391_msk_1.map emd_44391_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
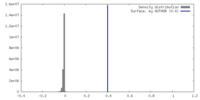
| 密度ヒストグラム |
-追加マップ: sharpened
| ファイル | emd_44391_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | sharpened | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
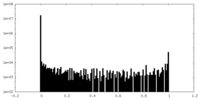
| 密度ヒストグラム |
-ハーフマップ: #2
| ファイル | emd_44391_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
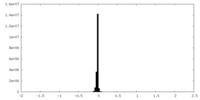
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_44391_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : ZBTB9 BTB domain filament
| 全体 | 名称: ZBTB9 BTB domain filament |
|---|---|
| 要素 |
|
-超分子 #1: ZBTB9 BTB domain filament
| 超分子 | 名称: ZBTB9 BTB domain filament / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Zinc finger and BTB domain-containing protein 9
| 分子 | 名称: Zinc finger and BTB domain-containing protein 9 / タイプ: protein_or_peptide / ID: 1 / コピー数: 14 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 19.387961 KDa |
| 組換発現 | 生物種:  Trichoplusia ni (イラクサキンウワバ) Trichoplusia ni (イラクサキンウワバ) |
| 配列 | 文字列: MDWSHPQFEK SAVGLNDIFE AQKIEWHEGG GGSGENLYFQ GGGRNPAPRT IQIEFPQHSS SLLESLNRHR LEGKFCDVSL LVQGRELRA HKAVLAAASP YFHDKLLLGD APRLTLPSVI EADAFEGLLQ LIYSGRLRLP LDALPAHLLV ASGLQMWQVV D QCSEILRE LETSGGGI UniProtKB: Zinc finger and BTB domain-containing protein 9 |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | らせん対称体再構成法 |
| 試料の集合状態 | filament |
- 試料調製
試料調製
| 濃度 | 0.95 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 緩衝液 | pH: 7.4 構成要素:
詳細: 50 mM HEPES/NaOH pH 7.4, 200 mM NaCl, 0.25 mM CHAPSO, 1 mM TCEP | |||||||||||||||
| グリッド | モデル: Quantifoil R1.2/1.3 / 材質: COPPER / メッシュ: 300 / 支持フィルム - 材質: CARBON / 支持フィルム - トポロジー: HOLEY / 支持フィルム - Film thickness: 12 / 前処理 - タイプ: GLOW DISCHARGE / 前処理 - 時間: 60 sec. / 前処理 - 雰囲気: AIR / 前処理 - 気圧: 0.039 kPa 詳細: Grids were glow-discharged for 60 s at 15-20 mA and 39 Pa | |||||||||||||||
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 90 % / チャンバー内温度: 283 K / 装置: LEICA EM GP 詳細: Grids were vitrified using a Leica EM GP plunge freezer operated at 90% humidity and 10 C with 10 s pre-blot, 3 s blot, 3 s post-blot.. | |||||||||||||||
| 詳細 | Elution fractions from Strep-tag affinity chromatography were dialyzed overnight against 50 mM HEPES/NaOH pH 7.4, 200 mM NaCl, 1 mM TCEP and concentrated by centrifugation. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TALOS ARCTICA |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 撮影したグリッド数: 1 / 実像数: 1137 / 平均露光時間: 4.5 sec. / 平均電子線量: 50.1 e/Å2 詳細: 1 movie (45 frames) was acquired per hole and stage position. |
| 電子線 | 加速電圧: 200 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 50.0 µm / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最大 デフォーカス(公称値): 2.0 µm / 最小 デフォーカス(公称値): 0.8 µm / 倍率(公称値): 36000 |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Talos Arctica / 画像提供: FEI Company |
+ 画像解析
画像解析
-原子モデル構築 1
| 初期モデル | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| 詳細 | Real-space refinement without local grid search |
| 精密化 | 空間: REAL / プロトコル: OTHER / 温度因子: 762 |
| 得られたモデル |  PDB-9b9v: |
 ムービー
ムービー コントローラー
コントローラー













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)