+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
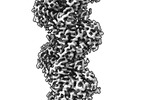
| Title | The structure of human cardiac F-actin | |||||||||
 Map data Map data | Final map used for model building. Post process and sharpening done in phenix using density modification scheme. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | actin / cardiac / human / sarcomere / CONTRACTILE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationactin filament-based movement / actin-myosin filament sliding / cardiac myofibril assembly / Formation of the dystrophin-glycoprotein complex (DGC) / cardiac muscle tissue morphogenesis / Striated Muscle Contraction / actomyosin structure organization / I band / RHOB GTPase cycle / microfilament motor activity ...actin filament-based movement / actin-myosin filament sliding / cardiac myofibril assembly / Formation of the dystrophin-glycoprotein complex (DGC) / cardiac muscle tissue morphogenesis / Striated Muscle Contraction / actomyosin structure organization / I band / RHOB GTPase cycle / microfilament motor activity / heart contraction / myosin binding / mesenchyme migration / skeletal muscle thin filament assembly / RHOA GTPase cycle / cardiac muscle contraction / actin filament organization / sarcomere / actin filament / filopodium / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / actin cytoskeleton / lamellipodium / cell body / response to ethanol / blood microparticle / hydrolase activity / response to xenobiotic stimulus / focal adhesion / positive regulation of gene expression / negative regulation of apoptotic process / glutamatergic synapse / extracellular space / extracellular exosome / ATP binding / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Doran MH / Sousa D / Rynkiewicz MJ / Lehman W / Cammarato A | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: iScience / Year: 2025 Journal: iScience / Year: 2025Title: The hypertrophic cardiomyopathy-associated A331P actin variant enhances basal contractile activity and elicits resting muscle dysfunction. Authors: Matthew H Doran / Michael J Rynkiewicz / Evan Despond / Meera C Viswanathan / Aditi Madan / Kripa Chitre / Axel J Fenwick / Duncan Sousa / William Lehman / John F Dawson / Anthony Cammarato /   Abstract: Previous studies aimed at defining the mechanistic basis of hypertrophic cardiomyopathy caused by A331P cardiac actin have reported conflicting results. The mutation is located along an actin surface ...Previous studies aimed at defining the mechanistic basis of hypertrophic cardiomyopathy caused by A331P cardiac actin have reported conflicting results. The mutation is located along an actin surface strand, proximal to residues that interact with tropomyosin. These F-actin-tropomyosin associations are vital for proper contractile inhibition. To help resolve disease pathogenesis, we implemented a multidisciplinary approach. Transgenic , expressing A331P actin, displayed skeletal muscle hypercontraction and elevated basal myocardial activity. A331P thin filaments, reconstituted using recombinant human cardiac actin, exhibited higher myosin-based sliding speeds, exclusively at low Ca concentrations. Cryo-EM-based reconstructions revealed no detectable A331P-related structural perturbations in F-actin. , however, the P331-containing actin surface strand was less mobile and established diminished van der Waal's attractive forces with tropomyosin, which correlated with greater variability in inhibitory tropomyosin positioning. Such mutation-induced effects potentially elevate resting contractile activity among our models and may stimulate pathology in patients. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44154.map.gz emd_44154.map.gz | 6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44154-v30.xml emd-44154-v30.xml emd-44154.xml emd-44154.xml | 24.2 KB 24.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_44154.png emd_44154.png | 85.1 KB | ||
| Filedesc metadata |  emd-44154.cif.gz emd-44154.cif.gz | 6.7 KB | ||
| Others |  emd_44154_additional_1.map.gz emd_44154_additional_1.map.gz emd_44154_additional_2.map.gz emd_44154_additional_2.map.gz emd_44154_half_map_1.map.gz emd_44154_half_map_1.map.gz emd_44154_half_map_2.map.gz emd_44154_half_map_2.map.gz | 78.3 MB 64.6 MB 65.2 MB 65.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44154 http://ftp.pdbj.org/pub/emdb/structures/EMD-44154 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44154 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44154 | HTTPS FTP |
-Validation report
| Summary document |  emd_44154_validation.pdf.gz emd_44154_validation.pdf.gz | 770.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_44154_full_validation.pdf.gz emd_44154_full_validation.pdf.gz | 770.3 KB | Display | |
| Data in XML |  emd_44154_validation.xml.gz emd_44154_validation.xml.gz | 11.7 KB | Display | |
| Data in CIF |  emd_44154_validation.cif.gz emd_44154_validation.cif.gz | 13 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44154 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44154 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44154 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44154 | HTTPS FTP |
-Related structure data
| Related structure data |  9b3rMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_44154.map.gz / Format: CCP4 / Size: 6.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44154.map.gz / Format: CCP4 / Size: 6.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final map used for model building. Post process and sharpening done in phenix using density modification scheme. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.058 Å | ||||||||||||||||||||||||||||||||||||
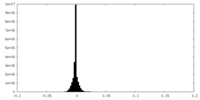
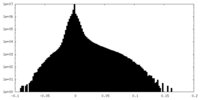
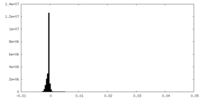
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Post-processed map in RELION.
| File | emd_44154_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Post-processed map in RELION. | ||||||||||||
| Projections & Slices |
| ||||||||||||
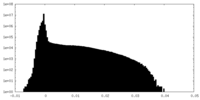
| Density Histograms |
-Additional map: Refinement from RELION without post-processing.
| File | emd_44154_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refinement from RELION without post-processing. | ||||||||||||
| Projections & Slices |
| ||||||||||||
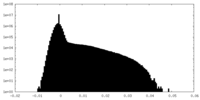
| Density Histograms |
-Half map: Half map 2.
| File | emd_44154_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2. | ||||||||||||
| Projections & Slices |
| ||||||||||||
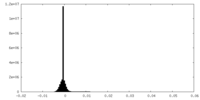
| Density Histograms |
-Half map: Half map 1.
| File | emd_44154_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1. | ||||||||||||
| Projections & Slices |
| ||||||||||||
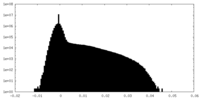
| Density Histograms |
- Sample components
Sample components
-Entire : Human cardiac F-actin
| Entire | Name: Human cardiac F-actin |
|---|---|
| Components |
|
-Supramolecule #1: Human cardiac F-actin
| Supramolecule | Name: Human cardiac F-actin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: ACTC was expressed in Sf21 insect cells, using recombinant baculoviruses, and purified via gelsolin affinity chromatography |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 420 KDa |
-Macromolecule #1: Actin, alpha cardiac muscle 1
| Macromolecule | Name: Actin, alpha cardiac muscle 1 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 42.07791 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MCDDEETTAL VCDNGSGLVK AGFAGDDAPR AVFPSIVGRP RHQGVMVGMG QKDSYVGDEA QSKRGILTLK YPIE(HIC)G IIT NWDDMEKIWH HTFYNELRVA PEEHPTLLTE APLNPKANRE KMTQIMFETF NVPAMYVAIQ AVLSLYASGR TTGIVLD SG DGVTHNVPIY ...String: MCDDEETTAL VCDNGSGLVK AGFAGDDAPR AVFPSIVGRP RHQGVMVGMG QKDSYVGDEA QSKRGILTLK YPIE(HIC)G IIT NWDDMEKIWH HTFYNELRVA PEEHPTLLTE APLNPKANRE KMTQIMFETF NVPAMYVAIQ AVLSLYASGR TTGIVLD SG DGVTHNVPIY EGYALPHAIM RLDLAGRDLT DYLMKILTER GYSFVTTAER EIVRDIKEKL CYVALDFENE MATAASSS S LEKSYELPDG QVITIGNERF RCPETLFQPS FIGMESAGIH ETTYNSIMKC DIDIRKDLYA NNVLSGGTTM YPGIADRMQ KEITALAPST MKIKIIAPPE RKYSVWIGGS ILASLSTFQQ MWISKQEYDE AGPSIVHRKC F UniProtKB: Actin, alpha cardiac muscle 1 |
-Macromolecule #2: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 2 / Number of copies: 3 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 3 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Details: 2 mmolL-1 Tris (pH 8), 0.2 mmolL-1 CaCl2, 0.2 mmolL-1 ATP, 0.5 mmolL-1 b-mercaptoethanol, 0.002% NaN3 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 8.0 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 27.93 Å Applied symmetry - Helical parameters - Δ&Phi: -166.48 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 133289 |
|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
| Startup model | Type of model: OTHER / Details: Featureless cylinder |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller








 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)