[English] 日本語
 Yorodumi
Yorodumi- EMDB-44133: filament of type 1 KD-mxyl miniature tau macrocycle derived from ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | filament of type 1 KD-mxyl miniature tau macrocycle derived from 4R tauopathic fold | |||||||||
 Map data Map data | filament of type 1 KD-mxyl miniature tau macrocycle derived from 4R tauopathic fold | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | tauopathies / neurodegenerative disorders / seed-competent miniature tau macrocycle / PROTEIN FIBRIL | |||||||||
| Function / homology |  Function and homology information Function and homology informationplus-end-directed organelle transport along microtubule / histone-dependent DNA binding / negative regulation of protein localization to mitochondrion / neurofibrillary tangle / microtubule lateral binding / axonal transport / tubulin complex / positive regulation of protein localization to synapse / phosphatidylinositol bisphosphate binding / generation of neurons ...plus-end-directed organelle transport along microtubule / histone-dependent DNA binding / negative regulation of protein localization to mitochondrion / neurofibrillary tangle / microtubule lateral binding / axonal transport / tubulin complex / positive regulation of protein localization to synapse / phosphatidylinositol bisphosphate binding / generation of neurons / rRNA metabolic process / axonal transport of mitochondrion / regulation of mitochondrial fission / axon development / regulation of microtubule-based movement / regulation of chromosome organization / central nervous system neuron development / intracellular distribution of mitochondria / minor groove of adenine-thymine-rich DNA binding / lipoprotein particle binding / microtubule polymerization / negative regulation of mitochondrial membrane potential / regulation of microtubule polymerization / dynactin binding / apolipoprotein binding / main axon / protein polymerization / axolemma / Caspase-mediated cleavage of cytoskeletal proteins / regulation of microtubule polymerization or depolymerization / negative regulation of mitochondrial fission / glial cell projection / neurofibrillary tangle assembly / positive regulation of axon extension / regulation of cellular response to heat / Activation of AMPK downstream of NMDARs / positive regulation of superoxide anion generation / positive regulation of protein localization / cellular response to brain-derived neurotrophic factor stimulus / supramolecular fiber organization / regulation of long-term synaptic depression / positive regulation of microtubule polymerization / cytoplasmic microtubule organization / synapse assembly / regulation of calcium-mediated signaling / somatodendritic compartment / axon cytoplasm / astrocyte activation / phosphatidylinositol binding / nuclear periphery / enzyme inhibitor activity / stress granule assembly / protein phosphatase 2A binding / regulation of microtubule cytoskeleton organization / cellular response to reactive oxygen species / microglial cell activation / Hsp90 protein binding / cellular response to nerve growth factor stimulus / protein homooligomerization / synapse organization / PKR-mediated signaling / regulation of synaptic plasticity / regulation of autophagy / SH3 domain binding / response to lead ion / microtubule cytoskeleton organization / memory / neuron projection development / cytoplasmic ribonucleoprotein granule / cell-cell signaling / single-stranded DNA binding / protein-folding chaperone binding / cellular response to heat / microtubule cytoskeleton / actin binding / growth cone / cell body / double-stranded DNA binding / microtubule binding / protein-macromolecule adaptor activity / sequence-specific DNA binding / amyloid fibril formation / dendritic spine / microtubule / learning or memory / neuron projection / membrane raft / negative regulation of gene expression / axon / neuronal cell body / DNA damage response / dendrite / protein kinase binding / enzyme binding / mitochondrion / DNA binding / RNA binding / extracellular region / identical protein binding / nucleus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Xu X / Angera JI / Rajewski HB / Jiang W / Del Valle RJ | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Chem / Year: 2025 Journal: Nat Chem / Year: 2025Title: Macrocyclic β-arch peptides that mimic the structure and function of disease-associated tau folds. Authors: Isaac J Angera / Xueyong Xu / Benjamin H Rajewski / Grace I Hallinan / Xiaoqi Zhang / Bernardino Ghetti / Ruben Vidal / Wen Jiang / Juan R Del Valle /  Abstract: Tauopathies are a class of neurodegenerative disorders that feature tau protein aggregates in the brain. Misfolded tau has the capacity to seed the fibrillization of soluble tau, leading to the prion- ...Tauopathies are a class of neurodegenerative disorders that feature tau protein aggregates in the brain. Misfolded tau has the capacity to seed the fibrillization of soluble tau, leading to the prion-like spread of aggregates. Within these filaments, tau protomers always exhibit a cross-β amyloid structure. However, distinct cross-β amyloid folds correlate with specific diseases. An understanding of how these conformations impact seeding activity remains elusive. Identifying the minimal epitopes required for transcellular propagation of tau aggregates represents a key step towards more relevant models of disease progression. Here we implement a diversity-oriented peptide macrocyclization approach towards miniature tau, or 'mini-tau', proteomimetics that can seed the aggregation of tau in engineered cells and primary neurons. Structural elucidation of one such seed-competent macrocycle reveals remarkable conformational congruence with core folds from patient-derived extracts of tau. The ability to impart β-arch form and function through peptide stapling has broad-ranging implications for the minimization and mimicry of pathological tau and other amyloid proteins that drive neurodegeneration. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44133.map.gz emd_44133.map.gz | 116.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44133-v30.xml emd-44133-v30.xml emd-44133.xml emd-44133.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_44133_fsc.xml emd_44133_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_44133.png emd_44133.png | 150 KB | ||
| Filedesc metadata |  emd-44133.cif.gz emd-44133.cif.gz | 5.3 KB | ||
| Others |  emd_44133_half_map_1.map.gz emd_44133_half_map_1.map.gz emd_44133_half_map_2.map.gz emd_44133_half_map_2.map.gz | 98.1 MB 98.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44133 http://ftp.pdbj.org/pub/emdb/structures/EMD-44133 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44133 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44133 | HTTPS FTP |
-Related structure data
| Related structure data |  9b3aMC  9dmeC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_44133.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44133.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | filament of type 1 KD-mxyl miniature tau macrocycle derived from 4R tauopathic fold | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.822 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map A
| File | emd_44133_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map B
| File | emd_44133_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : type 1 KD-mxyl miniature tau macrocycle derived from 4R tauopathi...
| Entire | Name: type 1 KD-mxyl miniature tau macrocycle derived from 4R tauopathic fold |
|---|---|
| Components |
|
-Supramolecule #1: type 1 KD-mxyl miniature tau macrocycle derived from 4R tauopathi...
| Supramolecule | Name: type 1 KD-mxyl miniature tau macrocycle derived from 4R tauopathic fold type: cell / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Microtubule-associated protein tau
| Macromolecule | Name: Microtubule-associated protein tau / type: protein_or_peptide / ID: 1 / Number of copies: 15 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.24264 KDa |
| Sequence | String: (ACE)CDNIKHVPG GGSVQIVYKP VC UniProtKB: Microtubule-associated protein tau |
-Macromolecule #2: SER-VAL-GLN-ILE-VAL-TYR-LYS
| Macromolecule | Name: SER-VAL-GLN-ILE-VAL-TYR-LYS / type: protein_or_peptide / ID: 2 / Number of copies: 15 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 836.995 Da |
| Sequence | String: SVQIVYK |
-Macromolecule #3: AMINO GROUP
| Macromolecule | Name: AMINO GROUP / type: ligand / ID: 3 / Number of copies: 15 / Formula: NH2 |
|---|---|
| Molecular weight | Theoretical: 16.023 Da |
| Chemical component information |  ChemComp-NH2: |
-Macromolecule #4: 1,3-dimethylbenzene
| Macromolecule | Name: 1,3-dimethylbenzene / type: ligand / ID: 4 / Number of copies: 15 / Formula: 8VH |
|---|---|
| Molecular weight | Theoretical: 106.165 Da |
| Chemical component information | 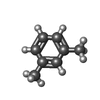 ChemComp-8VH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 4.5 mg/mL |
|---|---|
| Buffer | pH: 7.6 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 59.495 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: BACKBONE TRACE |
|---|---|
| Output model |  PDB-9b3a: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





































