[English] 日本語
 Yorodumi
Yorodumi- EMDB-44085: Pyrazinoate bound human URAT1 in the inward-facing state (site2) -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Pyrazinoate bound human URAT1 in the inward-facing state (site2) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SLC transporter / SLC22A family / uric acid / inward facing / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationDefective SLC22A12 causes renal hypouricemia 1 (RHUC1) / Organic anion transport by SLC22 transporters / renal urate salt excretion / urate transport / urate metabolic process / urate transmembrane transporter activity / organic anion transport / cellular homeostasis / monoatomic ion transport / PDZ domain binding ...Defective SLC22A12 causes renal hypouricemia 1 (RHUC1) / Organic anion transport by SLC22 transporters / renal urate salt excretion / urate transport / urate metabolic process / urate transmembrane transporter activity / organic anion transport / cellular homeostasis / monoatomic ion transport / PDZ domain binding / brush border membrane / cellular response to insulin stimulus / apical plasma membrane / response to xenobiotic stimulus / extracellular exosome / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Dai Y / Lee CH | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Res / Year: 2024 Journal: Cell Res / Year: 2024Title: Transport mechanism and structural pharmacology of human urate transporter URAT1. Authors: Yaxin Dai / Chia-Hsueh Lee /  Abstract: Urate is an endogenous product of purine metabolism in the liver. High urate levels in the blood lead to gout, a very common and painful inflammatory arthritis. Excreted urate is reabsorbed in the ...Urate is an endogenous product of purine metabolism in the liver. High urate levels in the blood lead to gout, a very common and painful inflammatory arthritis. Excreted urate is reabsorbed in the kidney mainly by URAT1 antiporter, a key target for anti-gout drugs. To uncover the mechanisms of urate transport and drug inhibition, we determined cryo-EM structures of human URAT1 with urate, counter anion pyrazinoate, or anti-gout drugs of different chemotypes - lesinurad, verinurad, and dotinurad. We captured the outward-to-inward transition of URAT1 during urate uptake, revealing that urate binds in a phenylalanine-rich pocket and engages with key gating residues to drive the transport cycle. In contrast to the single binding site for urate, pyrazinoate interacts with three distinct, functionally relevant sites within URAT1, a mechanism that has not yet been observed in other anion antiporters. In addition, we found that while all three drugs compete with substrates and halt the transport cycle, verinurad and dotinurad further hijack gating residues to achieve high potency. These insights advance our understanding of organic anion transport and provide a foundation for designing improved gout therapeutics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44085.map.gz emd_44085.map.gz | 203.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44085-v30.xml emd-44085-v30.xml emd-44085.xml emd-44085.xml | 14 KB 14 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_44085.png emd_44085.png | 82.8 KB | ||
| Filedesc metadata |  emd-44085.cif.gz emd-44085.cif.gz | 5.7 KB | ||
| Others |  emd_44085_half_map_1.map.gz emd_44085_half_map_1.map.gz emd_44085_half_map_2.map.gz emd_44085_half_map_2.map.gz | 200.7 MB 200.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44085 http://ftp.pdbj.org/pub/emdb/structures/EMD-44085 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44085 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44085 | HTTPS FTP |
-Validation report
| Summary document |  emd_44085_validation.pdf.gz emd_44085_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_44085_full_validation.pdf.gz emd_44085_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_44085_validation.xml.gz emd_44085_validation.xml.gz | 15.4 KB | Display | |
| Data in CIF |  emd_44085_validation.cif.gz emd_44085_validation.cif.gz | 18 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44085 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44085 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44085 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-44085 | HTTPS FTP |
-Related structure data
| Related structure data |  9b1nMC  9b1fC  9b1gC  9b1hC  9b1iC  9b1jC  9b1kC  9b1lC  9b1mC  9b1oC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_44085.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44085.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.649 Å | ||||||||||||||||||||||||||||||||||||
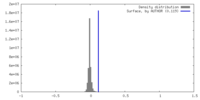
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_44085_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
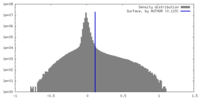
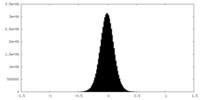
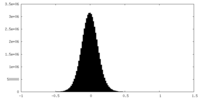
| Density Histograms |
-Half map: #1
| File | emd_44085_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
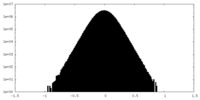
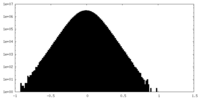
| Density Histograms |
- Sample components
Sample components
-Entire : Pyrazinoate bound human URAT1 in the inward-facing state (site2).
| Entire | Name: Pyrazinoate bound human URAT1 in the inward-facing state (site2). |
|---|---|
| Components |
|
-Supramolecule #1: Pyrazinoate bound human URAT1 in the inward-facing state (site2).
| Supramolecule | Name: Pyrazinoate bound human URAT1 in the inward-facing state (site2). type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Solute carrier family 22 member 12
| Macromolecule | Name: Solute carrier family 22 member 12 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 59.257402 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: FSELLDLVGG LGRFQVLQTM ALMVSIMWLC TQSMLENFSA AVPSHRCWAP LLDNSTAVST SLSPEALLAI SIPPGPNQRP HQCRRFRQP QWQLLDPNAT ATSWSEADTE PCVDGWVYDR SIFTSTIVAK WNLVCDSHAL KPMAQSIYLA GILVGAAACG P ASDRFGRR ...String: FSELLDLVGG LGRFQVLQTM ALMVSIMWLC TQSMLENFSA AVPSHRCWAP LLDNSTAVST SLSPEALLAI SIPPGPNQRP HQCRRFRQP QWQLLDPNAT ATSWSEADTE PCVDGWVYDR SIFTSTIVAK WNLVCDSHAL KPMAQSIYLA GILVGAAACG P ASDRFGRR LVLTWSYLQM AVMGTAAAFA PAFPVYCLFR FLLAFAVAGV MMNTGTLLME WTAARARPLV MTLNSLGFSF GH GLTAAVA YGVRDWTLLQ LVVSVPFFLC FLYSWWLPES ARWLIIKGKP DQALQELRKV ARINGHKEAK NLTIEVLMSS VKE EVASAK EPRSVLDLFC VPGLRFRTCI STLCWFAFGF TFFGLALDLQ ALGSNIFLLQ MFIGVVDIPA KMGALLLLSH LGRR PTLAA SLLLAGLCIL ANTLVPHEMG ALRSALAVLG LGGVGAAFTC ITIYSSELFP TVLRMTAVGL GQMAARGGAI LGPLV RLLG VHGPWLPLLV YGTVPVLSGL AALLLPETQS LPLPDTIQDV QNQAVKKATH GTLGNSVLKS TQF UniProtKB: Solute carrier family 22 member 12 |
-Macromolecule #2: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 1 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #3: PYRAZINE-2-CARBOXYLIC ACID
| Macromolecule | Name: PYRAZINE-2-CARBOXYLIC ACID / type: ligand / ID: 3 / Number of copies: 1 / Formula: VGL |
|---|---|
| Molecular weight | Theoretical: 124.097 Da |
| Chemical component information | 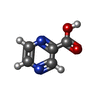 ChemComp-VGL: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Pretreatment - Type: PLASMA CLEANING |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 68.9 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 49989 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)