+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4293 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Hsp70:p53-TMGST:CHIP | |||||||||
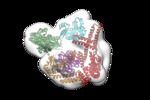
 Map data Map data | Hsp70:p53-TMGST:CHIP | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 15.0 Å | |||||||||
 Authors Authors | Quintana-Gallardo L / Martin-Benito J / Valpuesta JM | |||||||||
 Citation Citation |  Journal: Sci Rep / Year: 2019 Journal: Sci Rep / Year: 2019Title: The cochaperone CHIP marks Hsp70- and Hsp90-bound substrates for degradation through a very flexible mechanism. Authors: Lucía Quintana-Gallardo / Jaime Martín-Benito / Miguel Marcilla / Guadalupe Espadas / Eduard Sabidó / José María Valpuesta /  Abstract: Some molecular chaperones are involved not only in assisting the folding of proteins but also, given appropriate conditions, in their degradation. This is the case for Hsp70 and Hsp90 which, in ...Some molecular chaperones are involved not only in assisting the folding of proteins but also, given appropriate conditions, in their degradation. This is the case for Hsp70 and Hsp90 which, in concert with the cochaperone CHIP, direct their bound substrate to degradation through ubiquitination. We generated complexes between the chaperones (Hsp70 or Hsp90), the cochaperone CHIP and, as substrate, a p53 variant containing the GST protein (p53-TMGST). Both ternary complexes (Hsp70:p53-TMGST:CHIP and Hsp90:p53-TMGST:CHIP) ubiquitinated the substrate at a higher efficiency than in the absence of the chaperones. The 3D structures of the two complexes, obtained using a combination of cryoelectron microscopy and crosslinking mass spectrometry, showed the substrate located between the chaperone and the cochaperone, suggesting a ubiquitination mechanism in which the chaperone-bound substrate is presented to CHIP. These complexes are inherently flexible, which is important for the ubiquitination process. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4293.map.gz emd_4293.map.gz | 23.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4293-v30.xml emd-4293-v30.xml emd-4293.xml emd-4293.xml | 7.3 KB 7.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4293.png emd_4293.png | 179.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4293 http://ftp.pdbj.org/pub/emdb/structures/EMD-4293 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4293 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4293 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_4293.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4293.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Hsp70:p53-TMGST:CHIP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.8 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Hsp70:p53-TMGST:CHIP
| Entire | Name: Hsp70:p53-TMGST:CHIP |
|---|---|
| Components |
|
-Supramolecule #1: Hsp70:p53-TMGST:CHIP
| Supramolecule | Name: Hsp70:p53-TMGST:CHIP / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 200 kDa/nm |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 15.0 Å / Resolution method: FSC 0.33 CUT-OFF / Number images used: 25000 |
|---|---|
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: OTHER |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















